Acid Base Titration Lab Answer Key . 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. In equation 1, the acid is hcl (hydrochloric. To begin, check that 1 m naoh is selected for the burette,. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. What is the concentration of the hcl solution? Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: 2) you are titrating an acid into a base to determine the concentration. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid.
from www.numerade.com
In equation 1, the acid is hcl (hydrochloric. 2) you are titrating an acid into a base to determine the concentration. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the burette,. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. What is the concentration of the hcl solution? Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid.
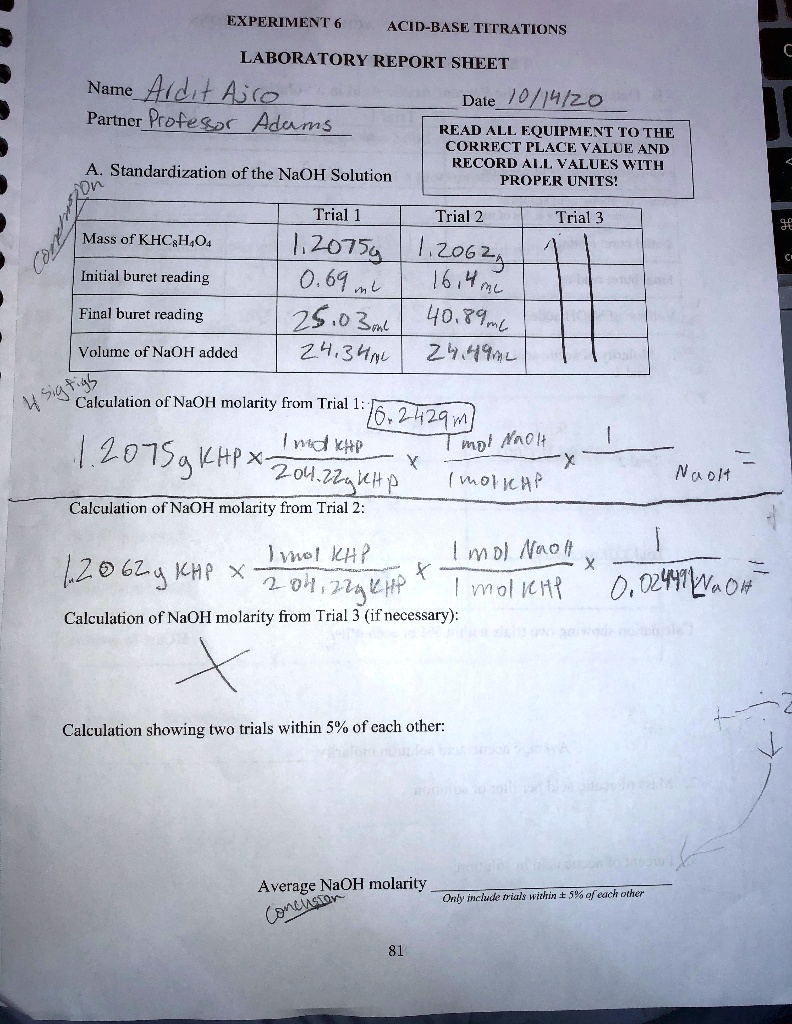
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET
Acid Base Titration Lab Answer Key To begin, check that 1 m naoh is selected for the burette,. What is the concentration of the hcl solution? Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. The analyte (titrand) is the solution. 2) you are titrating an acid into a base to determine the concentration. Titration is an analytical quantitative technique used to determine the concentration of a solute; In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In equation 1, the acid is hcl (hydrochloric. To begin, check that 1 m naoh is selected for the burette,.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 2) you are titrating an acid into a base to determine the concentration. To begin, check that 1 m naoh is selected for. Acid Base Titration Lab Answer Key.
From www.chegg.com
REPORT SHEET LAB AcidBase Titration 12 A. Concent... Acid Base Titration Lab Answer Key One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: 2) you are titrating an acid into a base to determine the concentration. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. The analyte (titrand). Acid Base Titration Lab Answer Key.
From www.myxxgirl.com
Acid Base Titration Lab Quiz Google Docs My XXX Hot Girl Acid Base Titration Lab Answer Key The analyte (titrand) is the solution. Titration is an analytical quantitative technique used to determine the concentration of a solute; If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. In equation 1, the acid is hcl (hydrochloric. 1) it takes 83 ml of a 0.45 m naoh. Acid Base Titration Lab Answer Key.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Acid Base Titration Lab Answer Key In equation 1, the acid is hcl (hydrochloric. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the burette,. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and. Acid Base Titration Lab Answer Key.
From www.studocu.com
AcidBase Titration Lab Part II Determination of Citric Acid in Acid Base Titration Lab Answer Key In equation 1, the acid is hcl (hydrochloric. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution. Titration is an analytical quantitative technique. Acid Base Titration Lab Answer Key.
From soetrust.org
2022 UPDATED!!! Acid base titration lab answers Soetrust Acid Base Titration Lab Answer Key In equation 1, the acid is hcl (hydrochloric. Titration is an analytical quantitative technique used to determine the concentration of a solute; What is the concentration of the hcl solution? If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. 2) you are titrating an acid into a. Acid Base Titration Lab Answer Key.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. To begin, check that 1 m naoh is selected for the burette,. 2) you are titrating an acid into a base to determine the concentration. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an. Acid Base Titration Lab Answer Key.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Answer Key What is the concentration of the hcl solution? Titration is an analytical quantitative technique used to determine the concentration of a solute; In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The analyte (titrand) is the solution. In equation 1, the acid is hcl (hydrochloric. 1) it takes 83 ml. Acid Base Titration Lab Answer Key.
From studyfinder.org
Mastering Acid Base Titration Your Comprehensive Worksheet Answer Key Acid Base Titration Lab Answer Key Titration is an analytical quantitative technique used to determine the concentration of a solute; To begin, check that 1 m naoh is selected for the burette,. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. In equation 1, the acid is hcl (hydrochloric. 2) you are titrating an acid into a. Acid Base Titration Lab Answer Key.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Answer Key Titration is an analytical quantitative technique used to determine the concentration of a solute; One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: The analyte (titrand) is the solution. What is the concentration of the hcl solution? In the titration gizmo, you will use indicators to show. Acid Base Titration Lab Answer Key.
From www.numerade.com
SOLVED REPORT SHEET LAB AcidBase Titration Concentration of Acetic Acid Base Titration Lab Answer Key 2) you are titrating an acid into a base to determine the concentration. The analyte (titrand) is the solution. To begin, check that 1 m naoh is selected for the burette,. Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic,. Acid Base Titration Lab Answer Key.
From www.studocu.com
Titration (Gizmo) Lab 201 Titration (Gizmo) Lab Vocabulary acid Acid Base Titration Lab Answer Key To begin, check that 1 m naoh is selected for the burette,. In equation 1, the acid is hcl (hydrochloric. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. 2) you are titrating an acid into a base to determine the concentration. Titration is an analytical quantitative technique used to. Acid Base Titration Lab Answer Key.
From www.numerade.com
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET Acid Base Titration Lab Answer Key The analyte (titrand) is the solution. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Titration is an analytical quantitative technique used to determine the concentration of a solute; One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water:. Acid Base Titration Lab Answer Key.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl (hydrochloric. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0. Acid Base Titration Lab Answer Key.
From www.docsity.com
Acid base titration lab answers Docsity Acid Base Titration Lab Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. The analyte (titrand) is the solution. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: What is the concentration of the hcl solution? 1) it. Acid Base Titration Lab Answer Key.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Acid Base Titration Lab Answer Key Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. What is the concentration of the hcl solution? One. Acid Base Titration Lab Answer Key.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Acid Base Titration Lab Answer Key 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. The analyte (titrand) is the solution. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. One type of titration uses a neutralization reaction, in which an acid and a base react. Acid Base Titration Lab Answer Key.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH Acid Base Titration Lab Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Titration is an analytical quantitative technique used to determine the concentration of a solute; To begin, check that 1. Acid Base Titration Lab Answer Key.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 20 1. Brand Acid Base Titration Lab Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. 2) you are titrating an acid into a base to determine the concentration. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1,. Acid Base Titration Lab Answer Key.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. 2). Acid Base Titration Lab Answer Key.
From exoqvrery.blob.core.windows.net
Introduction To Acid Base Titration Lab at Christopher Beatty blog Acid Base Titration Lab Answer Key One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The analyte (titrand) is the solution. In equation 1, the acid is hcl (hydrochloric. 2) you are titrating an. Acid Base Titration Lab Answer Key.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Acid Base Titration Lab Answer Key To begin, check that 1 m naoh is selected for the burette,. 2) you are titrating an acid into a base to determine the concentration. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. In the titration gizmo, you will use indicators to show how acids are. Acid Base Titration Lab Answer Key.
From www.studocu.com
15.2 acid base titration calculationsws answer key Name Studocu Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. 2) you are titrating an acid into a base to determine the concentration. The analyte (titrand) is the solution. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. In the titration gizmo,. Acid Base Titration Lab Answer Key.
From studylib.net
AcidBase Titration Lab.doc Acid Base Titration Lab Answer Key The analyte (titrand) is the solution. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Titration is an analytical quantitative technique used to determine the concentration of a. Acid Base Titration Lab Answer Key.
From worksheets.clipart-library.com
Name Acid Base Titration Lab Worksheet Titration Acid Base Titration Lab Answer Key Titration is an analytical quantitative technique used to determine the concentration of a solute; If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. In equation 1, the acid is hcl (hydrochloric. One type of titration uses a neutralization reaction, in which an acid and a base react. Acid Base Titration Lab Answer Key.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Acid Base Titration Lab Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. To begin, check that 1 m naoh is selected for the burette,. 2) you are titrating an acid into a base to determine the concentration. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize. Acid Base Titration Lab Answer Key.
From www.chegg.com
Solved AcidBase Titration Report Sheet Lab 20 B. Titration Acid Base Titration Lab Answer Key If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. In equation 1, the acid is hcl (hydrochloric. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. The analyte (titrand) is the solution. Titration is an analytical quantitative. Acid Base Titration Lab Answer Key.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Acid Base Titration Lab Answer Key 2) you are titrating an acid into a base to determine the concentration. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. What is the concentration of the hcl solution? Study. Acid Base Titration Lab Answer Key.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: What is the concentration of the hcl solution? In equation 1, the acid is hcl (hydrochloric. If 15.0 ml of. Acid Base Titration Lab Answer Key.
From mavink.com
Acid Base Titration Lab Procedure Acid Base Titration Lab Answer Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 2) you are titrating an acid into a base to determine the concentration. In equation 1, the acid is hcl (hydrochloric. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. One type. Acid Base Titration Lab Answer Key.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. The analyte (titrand) is the solution. In equation 1, the acid is hcl (hydrochloric. If 15.0 ml of 0.50 m naoh is used to neutralize 25.0 ml of hci, what is the molarity of the acid. What is the concentration of the. Acid Base Titration Lab Answer Key.
From www.studocu.com
Acid Base Titration Virtual Lab Nov 2020 Acid Base Titraion Deine the Acid Base Titration Lab Answer Key 2) you are titrating an acid into a base to determine the concentration. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Titration is an analytical quantitative technique used to determine the. Acid Base Titration Lab Answer Key.
From jeylennherdy.blogspot.com
Acid Base Titration Lab Report JeylennHerdy Acid Base Titration Lab Answer Key Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The analyte (titrand) is the solution. 2) you are titrating an acid into a base to determine the concentration. What is the concentration. Acid Base Titration Lab Answer Key.
From studylib.net
Lab Titration Acid Base Titration Lab Answer Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. 2) you are titrating an acid into a base to determine the concentration. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: The analyte (titrand) is the solution.. Acid Base Titration Lab Answer Key.
From mavink.com
Acid Base Titration Lab Procedure Acid Base Titration Lab Answer Key 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. 2) you are titrating an acid into a base to determine the concentration. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. If 15.0 ml of 0.50 m naoh is used to. Acid Base Titration Lab Answer Key.