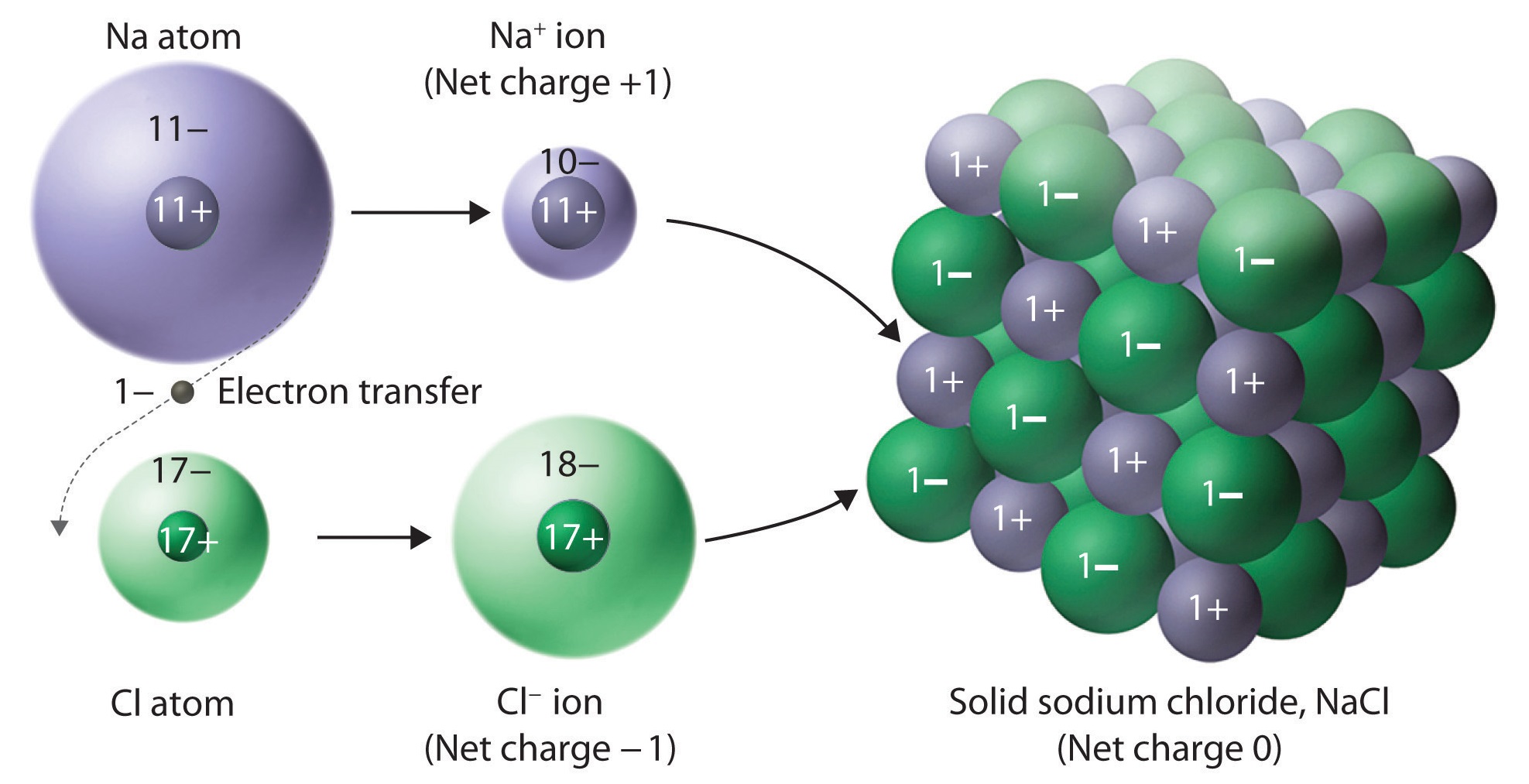
Does S Form A Negative Ion . A nitrogen atom must gain three electrons to have the same number of electrons as an atom. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. The ions formed are negative, because they. The atoms in chemical compounds are held together by attractive. A number of processes are. Ionic compounds usually form hard crystalline solids with high melting points. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Which of these ions is not likely to form? It achieves octet by gaining. Nonmetals form negative ions (anions). Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations).
from chem.libretexts.org
Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). The atoms in chemical compounds are held together by attractive. Nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an atom. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Ionic compounds usually form hard crystalline solids with high melting points. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. Which of these ions is not likely to form? A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. The ions formed are negative, because they.
Ionic Solids Chemistry LibreTexts
Does S Form A Negative Ion Which of these ions is not likely to form? It achieves octet by gaining. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Nonmetals form negative ions (anions). The ions formed are negative, because they. A nitrogen atom must gain three electrons to have the same number of electrons as an atom. A number of processes are. Ionic compounds usually form hard crystalline solids with high melting points. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). Which of these ions is not likely to form? The atoms in chemical compounds are held together by attractive. S 3− n 3− answer (a) s is in group 6a and has six valence electrons.
From klaflxeha.blob.core.windows.net
Will S Form A Negative Ion at Keith Coughlin blog Does S Form A Negative Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. It achieves octet by gaining. The basic principle of negative ion formation is to attach an extra electron to a neutral. Does S Form A Negative Ion.
From www.forygen.com
NEGATIVE ION FORYGEN Negative Ions Does S Form A Negative Ion A number of processes are. Ionic compounds usually form hard crystalline solids with high melting points. Nonmetals form negative ions (anions). S 3− n 3− answer (a) s is in group 6a and has six valence electrons. The atoms in chemical compounds are held together by attractive. A cation (a positive ion) forms when a neutral atom loses one or. Does S Form A Negative Ion.
From www.youtube.com
What is an Ion? YouTube Does S Form A Negative Ion It achieves octet by gaining. A number of processes are. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. Which of these ions is not likely to form? The atoms. Does S Form A Negative Ion.
From www.numerade.com
SOLVED Complete the table with the positive ion negative ion, and the Does S Form A Negative Ion The atoms in chemical compounds are held together by attractive. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. A number of processes are. It achieves octet by gaining. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Does S Form A Negative Ion.
From elanra.com
All about positive and negative ions? Does S Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an atom. A number of processes are. Nonmetals form negative ions (anions). It achieves octet by gaining. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. The ions formed are negative, because they.. Does S Form A Negative Ion.
From www.nagwa.com
Question Video Describing the Attraction between the Ions of an Ionic Does S Form A Negative Ion The atoms in chemical compounds are held together by attractive. A number of processes are. Ionic compounds usually form hard crystalline solids with high melting points. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more. Does S Form A Negative Ion.
From lavabene.com
Negative ION's Houston Natural Detox Spa Holistic Wellness and Healing Does S Form A Negative Ion It achieves octet by gaining. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Ionic compounds usually form hard crystalline solids with high melting points. The atoms in. Does S Form A Negative Ion.
From studylambdacism.z21.web.core.windows.net
Do Metals Form Positive Or Negative Ions Does S Form A Negative Ion The ions formed are negative, because they. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Nonmetals form negative ions (anions). Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). Ionic compounds usually form hard crystalline solids. Does S Form A Negative Ion.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Does S Form A Negative Ion The atoms in chemical compounds are held together by attractive. A number of processes are. The ions formed are negative, because they. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become. Does S Form A Negative Ion.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Does S Form A Negative Ion It achieves octet by gaining. The atoms in chemical compounds are held together by attractive. A nitrogen atom must gain three electrons to have the same number of electrons as an atom. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. A cation (a positive ion) forms when. Does S Form A Negative Ion.
From ionspecphilippines.com
Negative Ion IONSPEC Does S Form A Negative Ion S 3− n 3− answer (a) s is in group 6a and has six valence electrons. The ions formed are negative, because they. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its. Does S Form A Negative Ion.
From socratic.org
What happens if an atom is not neutral? Socratic Does S Form A Negative Ion It achieves octet by gaining. The ions formed are negative, because they. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. A nitrogen atom must gain three electrons to have the same number of electrons as an atom. Metal atoms become more stable when they lose the electron (s) in their outer shell,. Does S Form A Negative Ion.
From deborahkruwhogan.blogspot.com
Which Is Most Likely to Form a Negative Ion DeborahkruwHogan Does S Form A Negative Ion A number of processes are. The ions formed are negative, because they. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. S 3− n 3− answer (a) s is in. Does S Form A Negative Ion.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Does S Form A Negative Ion S 3− n 3− answer (a) s is in group 6a and has six valence electrons. A number of processes are. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Does S Form A Negative Ion.
From tomi.digital
TOMi.digital Negative form. Present tense. Does S Form A Negative Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. A number of processes are. S 3− n 3− answer (a) s is in group 6a and has six valence electrons.. Does S Form A Negative Ion.
From healthylifedigest.blogspot.com
HealthyLifeDigest Negative ions, healthy life's vitamins of the air. Does S Form A Negative Ion Which of these ions is not likely to form? A nitrogen atom must gain three electrons to have the same number of electrons as an atom. The atoms in chemical compounds are held together by attractive. A number of processes are. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Does S Form A Negative Ion.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Does S Form A Negative Ion Which of these ions is not likely to form? The atoms in chemical compounds are held together by attractive. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). Nonmetals form negative ions (anions). A number of processes are. The basic principle of negative ion formation is to attach an. Does S Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does S Form A Negative Ion The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Which of these ions is not likely to form? A number of processes are. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). Nonmetals form negative ions (anions).. Does S Form A Negative Ion.
From ar.inspiredpencil.com
Periodic Table With Positive And Negative Charge Does S Form A Negative Ion The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Which of these ions is not likely to form? Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). Nonmetals form negative ions (anions). The ions formed are negative,. Does S Form A Negative Ion.
From loejtufzw.blob.core.windows.net
Which Element Can Form A Negative Ion Of The Type X3 at Chris Cohen blog Does S Form A Negative Ion The atoms in chemical compounds are held together by attractive. The ions formed are negative, because they. Ionic compounds usually form hard crystalline solids with high melting points. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one. Does S Form A Negative Ion.
From joieueami.blob.core.windows.net
Model Of An Ion at Juanita Jenkins blog Does S Form A Negative Ion Nonmetals form negative ions (anions). It achieves octet by gaining. A nitrogen atom must gain three electrons to have the same number of electrons as an atom. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. Ionic compounds usually form hard crystalline solids with high melting points. The atoms in chemical compounds are. Does S Form A Negative Ion.
From ar.inspiredpencil.com
What Is Ion Does S Form A Negative Ion It achieves octet by gaining. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). A number of processes are. Nonmetals form negative ions (anions). Which of these ions is not likely to form? The basic principle of negative ion formation is to attach an extra electron to a neutral. Does S Form A Negative Ion.
From klaflxeha.blob.core.windows.net
Will S Form A Negative Ion at Keith Coughlin blog Does S Form A Negative Ion Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. The ions formed. Does S Form A Negative Ion.
From www.tes.com
AQA ion tests revision notes bundle, positive and negative ions Does S Form A Negative Ion A number of processes are. A nitrogen atom must gain three electrons to have the same number of electrons as an atom. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence. Does S Form A Negative Ion.
From deborahkruwhogan.blogspot.com
Which Is Most Likely to Form a Negative Ion DeborahkruwHogan Does S Form A Negative Ion A number of processes are. A nitrogen atom must gain three electrons to have the same number of electrons as an atom. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion. Does S Form A Negative Ion.
From kimcampion.com
Anion_Formation Does S Form A Negative Ion Nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an atom. The ions formed are negative, because they. Ionic compounds usually form hard crystalline solids with high melting points. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and. Does S Form A Negative Ion.
From www.nagwa.com
Question Video Comparing a Positive Ion to a Neutral Atom Nagwa Does S Form A Negative Ion Nonmetals form negative ions (anions). The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. Which of these ions is not likely to form? A cation (a positive ion) forms when a neutral. Does S Form A Negative Ion.
From www.sliderbase.com
Formula of Ionic Compounds Does S Form A Negative Ion The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. Which of these ions is not likely to form? The atoms in chemical compounds are held together by attractive. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations).. Does S Form A Negative Ion.
From www.yourdictionary.com
Ion Examples With Positive & Negative Charges YourDictionary Does S Form A Negative Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction.. Does S Form A Negative Ion.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Does S Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an atom. Nonmetals form negative ions (anions). A number of processes are. The ions formed are negative, because they. S 3− n 3− answer (a) s is in group 6a and has six valence electrons. Metal atoms become more stable when they lose the electron. Does S Form A Negative Ion.
From kladkrsvl.blob.core.windows.net
Does Boron Form A Negative Ion at Marilyn Ahner blog Does S Form A Negative Ion The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. The ions formed are negative, because they. Nonmetals form negative ions (anions). Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). S 3− n 3− answer (a) s. Does S Form A Negative Ion.
From www.expii.com
IonDipole Forces — Definition & Overview Expii Does S Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an atom. The atoms in chemical compounds are held together by attractive. Metal atoms become more stable when they lose the electron (s) in their outer shell, and become positive ions (cations). A cation (a positive ion) forms when a neutral atom loses one or. Does S Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does S Form A Negative Ion Ionic compounds usually form hard crystalline solids with high melting points. Nonmetals form negative ions (anions). A number of processes are. Which of these ions is not likely to form? The basic principle of negative ion formation is to attach an extra electron to a neutral atom by an exothermic reaction. A nitrogen atom must gain three electrons to have. Does S Form A Negative Ion.
From gretchenmeowlogan.blogspot.com
What is an Ion Does S Form A Negative Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. Nonmetals form negative ions (anions). It achieves octet by gaining. Ionic compounds usually form hard crystalline solids with high melting points.. Does S Form A Negative Ion.
From exyeaqpsg.blob.core.windows.net
Ion Exchange For Dummies at Ashley Glynn blog Does S Form A Negative Ion A number of processes are. Ionic compounds usually form hard crystalline solids with high melting points. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. Which of these ions is. Does S Form A Negative Ion.