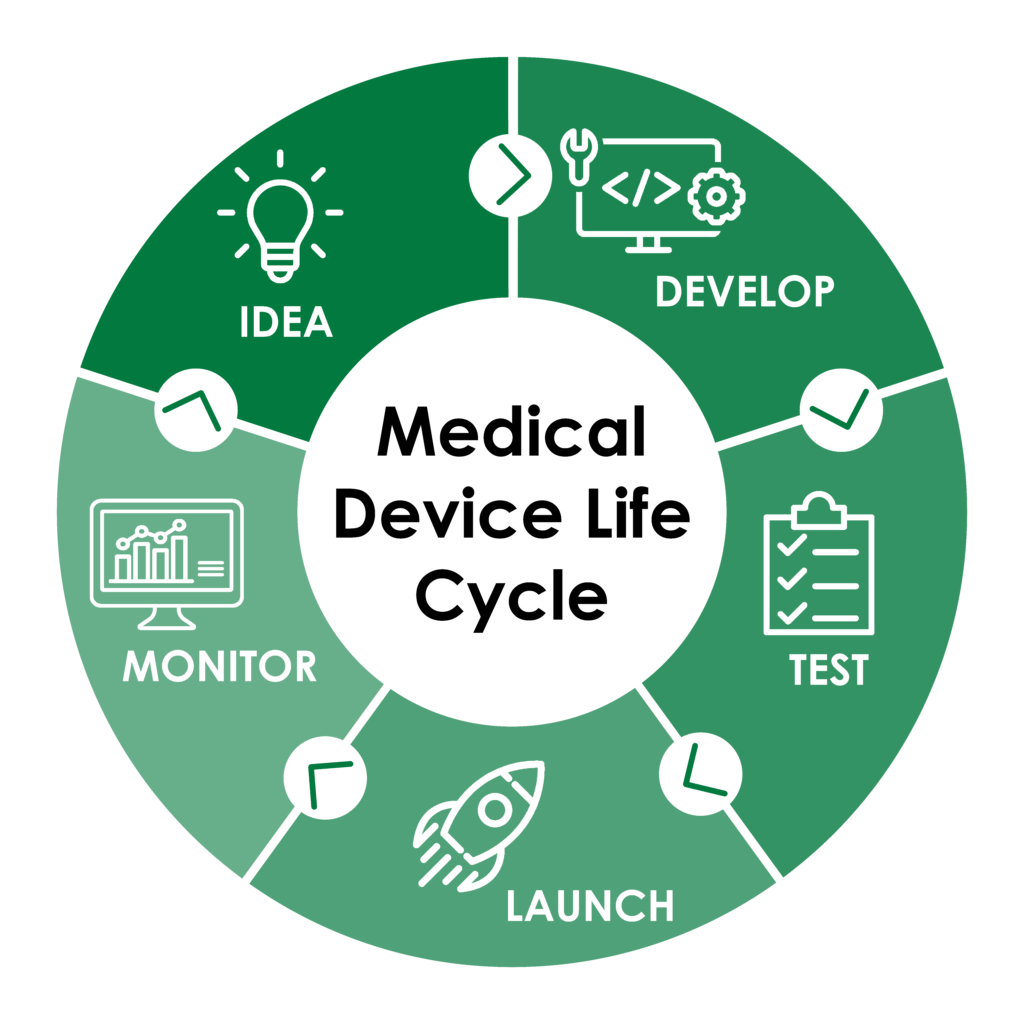
What Is Medical Device Life Cycle . design and development and phased life cycle of iso 14971. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. The life cycle model shown below encompasses the device’s. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at.
from enterprisepeak.com
this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. The life cycle model shown below encompasses the device’s. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. design and development and phased life cycle of iso 14971.
Medical Devices from Concept to Commercialization Enterprise Peak
What Is Medical Device Life Cycle this article introduces what the medical device life cycle means and how it affects the design of new medical devices. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. The life cycle model shown below encompasses the device’s. design and development and phased life cycle of iso 14971. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides.
From medicaldevicemktgblog.com
Six Critical Differences with Life Cycle Planning for Medical Devices What Is Medical Device Life Cycle total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in. What Is Medical Device Life Cycle.
From www.researchgate.net
Medical device lifecycle management (see online version for colours What Is Medical Device Life Cycle design and development and phased life cycle of iso 14971. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. while a medical device should be developed to meet the. What Is Medical Device Life Cycle.
From mavink.com
Medical Device Life Cycle Phases What Is Medical Device Life Cycle this article introduces what the medical device life cycle means and how it affects the design of new medical devices. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. The life cycle model shown below encompasses the device’s. this journey, known as the medical device life cycle, is. What Is Medical Device Life Cycle.
From quality.eleapsoftware.com
Understanding the Medical Device Life Cycle Navigating Stages with What Is Medical Device Life Cycle the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three. What Is Medical Device Life Cycle.
From www.qmswrapper.com
What is medical devices lifecycle qmsWrapper What Is Medical Device Life Cycle the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. The life cycle model shown below encompasses the device’s. this article introduces what the medical device life. What Is Medical Device Life Cycle.
From mdrsllc.com
Device Development Life Cycle What Is Medical Device Life Cycle total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in. What Is Medical Device Life Cycle.
From mavink.com
Mobile Device Life Cycle What Is Medical Device Life Cycle design and development and phased life cycle of iso 14971. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. this journey, known as the medical device life. What Is Medical Device Life Cycle.
From mavink.com
Medical Device Life Cycle Phases What Is Medical Device Life Cycle design and development and phased life cycle of iso 14971. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. invasive, or class iii,. What Is Medical Device Life Cycle.
From www.researchgate.net
Medical device lifecycle management (see online version for colours What Is Medical Device Life Cycle while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical.. What Is Medical Device Life Cycle.
From operonstrategist.com
5 Phases Of Medical Device Development (Step By Step Process) Operon What Is Medical Device Life Cycle this article introduces what the medical device life cycle means and how it affects the design of new medical devices. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. The life cycle model shown below encompasses the device’s. design and development and phased life cycle. What Is Medical Device Life Cycle.
From mavink.com
Medical Device Life Cycle Phases What Is Medical Device Life Cycle this article introduces what the medical device life cycle means and how it affects the design of new medical devices. design and development and phased life cycle of iso 14971. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this journey, known as. What Is Medical Device Life Cycle.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 What Is Medical Device Life Cycle this article introduces what the medical device life cycle means and how it affects the design of new medical devices. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. while a medical device should be developed to meet the requirements for its intended use, there. What Is Medical Device Life Cycle.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog What Is Medical Device Life Cycle while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. the effective management of medical devices throughout the medical device life cycle is a crucial. What Is Medical Device Life Cycle.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide What Is Medical Device Life Cycle while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. this. What Is Medical Device Life Cycle.
From www.ni.com
Easing the Medical Device Life Cycle with Virtual Instrumentation What Is Medical Device Life Cycle while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. design and development and phased life cycle of iso 14971. the effective management of medical devices throughout the medical. What Is Medical Device Life Cycle.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development What Is Medical Device Life Cycle invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this journey, known as the medical device life cycle, is. What Is Medical Device Life Cycle.
From www.vrogue.co
Understanding The Medical Device Product Lifecycle Dy vrogue.co What Is Medical Device Life Cycle design and development and phased life cycle of iso 14971. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this article introduces what the medical. What Is Medical Device Life Cycle.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 What Is Medical Device Life Cycle this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. while a medical device should be developed to meet the requirements. What Is Medical Device Life Cycle.
From www.joharidigital.com
Understanding The 7 Phases of Medical Device Development & Manufacturing What Is Medical Device Life Cycle this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. design and development and phased life cycle of iso 14971. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. this article introduces what the medical device. What Is Medical Device Life Cycle.
From www.presentationeze.com
Medical Device Validation Life Cycle PresentationEZE What Is Medical Device Life Cycle this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. The life cycle model shown below encompasses the device’s. total product life cycle approach strengthens cdrh’s focus on. What Is Medical Device Life Cycle.
From quality.eleapsoftware.com
Medical Device Life Cycle A Comprehensive Guide eLeaP What Is Medical Device Life Cycle invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. design and development and phased life cycle of iso 14971. this article introduces what the medical. What Is Medical Device Life Cycle.
From toolbox.eupati.eu
Making a medicine. Step 10 Lifecycle management EUPATI Toolbox What Is Medical Device Life Cycle design and development and phased life cycle of iso 14971. The life cycle model shown below encompasses the device’s. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. while a. What Is Medical Device Life Cycle.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co What Is Medical Device Life Cycle total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. design and development and phased life cycle of iso 14971. invasive, or class iii, medical devices (such as pacemakers,. What Is Medical Device Life Cycle.
From mavink.com
Mobile Device Life Cycle What Is Medical Device Life Cycle invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. The life cycle model shown below encompasses the device’s. total product life cycle approach. What Is Medical Device Life Cycle.
From www.slideshare.net
Presentation Life cycle of medical devices What Is Medical Device Life Cycle the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. this article introduces what the medical device life cycle means and how it affects the design of new. What Is Medical Device Life Cycle.
From english.igj.nl
Our supervision of medical technology Medical Technology Health and What Is Medical Device Life Cycle this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. design and development and phased life cycle of iso 14971. the effective management of medical devices throughout the medical device. What Is Medical Device Life Cycle.
From www.vrogue.co
Life Cycle Of A Medical Device Custom University Pape vrogue.co What Is Medical Device Life Cycle invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. The life cycle model shown below encompasses the device’s. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. this article. What Is Medical Device Life Cycle.
From enterprisepeak.com
Medical Devices from Concept to Commercialization Enterprise Peak What Is Medical Device Life Cycle this article introduces what the medical device life cycle means and how it affects the design of new medical devices. The life cycle model shown below encompasses the device’s. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. this journey, known as the medical device life cycle, is. What Is Medical Device Life Cycle.
From www.vrogue.co
Understanding The Medical Device Product Lifecycle Dy vrogue.co What Is Medical Device Life Cycle while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. total product life cycle approach strengthens cdrh’s focus on the safety and effectiveness of medical. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in. What Is Medical Device Life Cycle.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide What Is Medical Device Life Cycle this article introduces what the medical device life cycle means and how it affects the design of new medical devices. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. design and development and phased life cycle of iso 14971. this. What Is Medical Device Life Cycle.
From www.researchgate.net
Medical device lifecycle management (see online version for colours What Is Medical Device Life Cycle invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. The life cycle model shown below encompasses the device’s. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this. What Is Medical Device Life Cycle.
From aganamed.com
Educational Literature AganaMed LLC What Is Medical Device Life Cycle invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. the effective management of medical devices throughout the medical device life cycle is a crucial process that provides. this article introduces what the medical device life cycle means and how it affects. What Is Medical Device Life Cycle.
From 24x7mag.com
The Medical Device Security Life Cycle 24x7 What Is Medical Device Life Cycle this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. while a medical device should be developed to meet the requirements for its intended use, there are some general steps in the. this article introduces what the medical device life cycle means and how it affects. What Is Medical Device Life Cycle.
From www.greenlight.guru
Medical Device Development [Understanding the 5 Phases] What Is Medical Device Life Cycle invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. The life cycle model shown below encompasses the device’s. this journey, known as the medical device life cycle, is a comprehensive process that ensures safety, efficacy, and regulatory compliance at. this article. What Is Medical Device Life Cycle.
From www.pacific-research.com
Understanding the Noninvasive Medical Device Product Life Cycle What Is Medical Device Life Cycle design and development and phased life cycle of iso 14971. this article introduces what the medical device life cycle means and how it affects the design of new medical devices. invasive, or class iii, medical devices (such as pacemakers, stents, and artificial joints) face a much more stringent process in each of the three areas. The life. What Is Medical Device Life Cycle.