Acetic Acid Naoh Titration Equivalence Point . The term equivalence point means that the solutions have been mixed in exactly the right. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The term neutral point is best avoided. When used for pickling, the acetic acid content can be. The only thing in solution is its conjugate. the equivalence point occurs when equal moles of acid react with equal moles of base. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The mmol ch 3 cooh: Titration curve (acetic acid and naoh). the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3).
from www.numerade.com
The term neutral point is best avoided. The mmol ch 3 cooh: The term equivalence point means that the solutions have been mixed in exactly the right. the equivalence point occurs when equal moles of acid react with equal moles of base. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). The only thing in solution is its conjugate. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. When used for pickling, the acetic acid content can be. Titration curve (acetic acid and naoh).
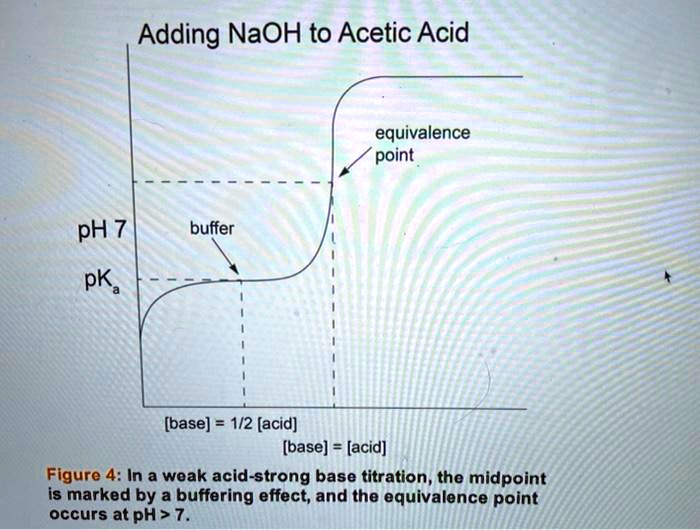
SOLVED Adding NaOH to Acetic Acid equivalence point pH 7 buffer pK
Acetic Acid Naoh Titration Equivalence Point When used for pickling, the acetic acid content can be. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The term equivalence point means that the solutions have been mixed in exactly the right. Titration curve (acetic acid and naoh). The mmol ch 3 cooh: When used for pickling, the acetic acid content can be. The term neutral point is best avoided. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the equivalence point occurs when equal moles of acid react with equal moles of base. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). The only thing in solution is its conjugate.
From byjus.com
25 ml of 0.1 M acetic acid is titrated with 0.1NaoH solution. The pH of Acetic Acid Naoh Titration Equivalence Point the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The term neutral point is best avoided. The mmol ch 3 cooh: The only thing in solution is its conjugate. Titration curve (acetic acid and naoh). the equivalence point occurs when equal moles of acid react. Acetic Acid Naoh Titration Equivalence Point.
From people.bu.edu
John Straub's lecture notes Acetic Acid Naoh Titration Equivalence Point The only thing in solution is its conjugate. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Titration curve (acetic acid and naoh). The term equivalence point means that the solutions have been mixed in exactly the right. When used for pickling, the acetic acid content can. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED29. 25 mL of 0.10 M acetic acid is titrated with 0.10 M NaOH Acetic Acid Naoh Titration Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the right. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3).. Acetic Acid Naoh Titration Equivalence Point.
From schoolbag.info
ACIDBASE TITRATIONS ADDITIONAL ASPECTS OF AQUEOUS EQUILIBRIA Acetic Acid Naoh Titration Equivalence Point in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the right. when naoh is added to acetic acid the equivalence point occurs at a ph of. Acetic Acid Naoh Titration Equivalence Point.
From exyuokede.blob.core.windows.net
Titration Graph Buffer at Mark Paras blog Acetic Acid Naoh Titration Equivalence Point in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The only thing in solution is its conjugate. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). Titration curve (acetic acid and naoh). illustrations. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED When examining the titrations of hydrochloric acid and acetic Acetic Acid Naoh Titration Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the right. the equivalence point occurs when equal moles of acid react with equal moles of base. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. when naoh is added to acetic. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED "Titrations A 50.00 ml aliquote of 0.0500 M HCI requires NaOH Acetic Acid Naoh Titration Equivalence Point illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The term equivalence point means that the solutions have been mixed in exactly the right. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Calculate the pH at the equivalence point in a 0.10 M acetic Acetic Acid Naoh Titration Equivalence Point When used for pickling, the acetic acid content can be. The mmol ch 3 cooh: the equivalence point occurs when equal moles of acid react with equal moles of base. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the right. in this experiment, a technique known. Acetic Acid Naoh Titration Equivalence Point.
From www.youtube.com
Titration Acetic Acid with NaOH YouTube Acetic Acid Naoh Titration Equivalence Point The term neutral point is best avoided. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. the equivalence point occurs when equal. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
If 2.50 mL of vinegar requires 34.9 mL of 0.0960M NaOH to reach Acetic Acid Naoh Titration Equivalence Point when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). The term equivalence point means that the solutions have been mixed in exactly the right. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v.. Acetic Acid Naoh Titration Equivalence Point.
From www.researchgate.net
Titration curve of acetic acid at 30 °C (Ve = equivalence volume Acetic Acid Naoh Titration Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the right. When used for pickling, the acetic acid content can be. The mmol ch 3 cooh: The only thing in solution is its conjugate. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar.. Acetic Acid Naoh Titration Equivalence Point.
From www.chegg.com
Solved Titration curve for the Titration of of 25.0 mL of Acetic Acid Naoh Titration Equivalence Point the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. the equivalence point occurs when equal moles of acid react with equal moles of base. The only thing in solution is its conjugate. Titration curve (acetic acid and naoh). The term equivalence point means that the. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Adding NaOH to Acetic Acid equivalence point pH 7 buffer pK Acetic Acid Naoh Titration Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the right. When used for pickling, the acetic acid content can be. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The term neutral point is best avoided. when naoh is. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Determine the pH during the titration of 66.3 mL of 0.483 M Acetic Acid Naoh Titration Equivalence Point in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). The mmol ch 3 cooh: Titration curve (acetic acid and naoh). The term neutral point is. Acetic Acid Naoh Titration Equivalence Point.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Acetic Acid Naoh Titration Equivalence Point The mmol ch 3 cooh: When used for pickling, the acetic acid content can be. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. the equivalence point occurs when equal moles of acid react with equal moles of base. when naoh is added to. Acetic Acid Naoh Titration Equivalence Point.
From giolipqwa.blob.core.windows.net
Titration Curve Of Nh3 And Hcl at Huntington blog Acetic Acid Naoh Titration Equivalence Point The mmol ch 3 cooh: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). the equivalence point occurs when equal moles of acid react. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED A student titrates 25.0 mL of 0.15 Macetic acid with 0.15 M Acetic Acid Naoh Titration Equivalence Point illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. in this experiment, a technique known as a titration will be used. Acetic Acid Naoh Titration Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Acetic Acid Naoh Titration Equivalence Point The mmol ch 3 cooh: when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). Titration curve (acetic acid and naoh). When used for pickling, the acetic acid content can be. in this experiment, a technique known as a titration will be used to determine the concentration. Acetic Acid Naoh Titration Equivalence Point.
From www.transtutors.com
(Solved) Acetic Acid Titrated With NaOH 10 8 ?? 6 4 0 2 6 8 Volume Of Acetic Acid Naoh Titration Equivalence Point The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the right. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The mmol ch 3 cooh: in this experiment, a technique known as a titration. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Consider the titration curve below for the titration of oxalic Acetic Acid Naoh Titration Equivalence Point Titration curve (acetic acid and naoh). in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. When used for pickling, the acetic acid content can be. the equivalence point occurs when equal moles of acid react with equal moles of base. the acetic acid content of. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED Model Titration Curves involving Polyprotic acids Oxalic acid Acetic Acid Naoh Titration Equivalence Point the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The only thing in solution is its conjugate. Titration curve (acetic acid and naoh). The term neutral point is best avoided. When used for pickling, the acetic acid content can be. the equivalence point occurs when. Acetic Acid Naoh Titration Equivalence Point.
From staff.buffalostate.edu
AcidBase Reactions Acetic Acid Naoh Titration Equivalence Point The only thing in solution is its conjugate. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. Titration curve (acetic acid and naoh). The term neutral point is best avoided. The mmol ch 3 cooh: When used for pickling, the acetic acid content can be.. Acetic Acid Naoh Titration Equivalence Point.
From fyoizufqj.blob.core.windows.net
Equivalence Point Amino Acid Titration at Christine Grosso blog Acetic Acid Naoh Titration Equivalence Point in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The term equivalence point means that the solutions have been mixed in exactly the. Acetic Acid Naoh Titration Equivalence Point.
From www.slideshare.net
Water And Buffers Acetic Acid Naoh Titration Equivalence Point Titration curve (acetic acid and naoh). The mmol ch 3 cooh: The only thing in solution is its conjugate. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). The term equivalence point means that the solutions have been mixed in exactly the right. illustrations showing the. Acetic Acid Naoh Titration Equivalence Point.
From mavink.com
Titration Curve Of Acetic Acid Acetic Acid Naoh Titration Equivalence Point The term equivalence point means that the solutions have been mixed in exactly the right. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3).. Acetic Acid Naoh Titration Equivalence Point.
From socratic.org
A "25.0mL" sample of "0.150mol L"^(1) acetic acid is titrated with a Acetic Acid Naoh Titration Equivalence Point illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The term equivalence point means that the solutions have been mixed in exactly the right. When used for pickling, the acetic acid content can be. Titration curve (acetic acid and naoh). the acetic acid content. Acetic Acid Naoh Titration Equivalence Point.
From www.chegg.com
Solved Reaction Equation, Acetic Acid with NaOH An acetic Acetic Acid Naoh Titration Equivalence Point When used for pickling, the acetic acid content can be. the equivalence point occurs when equal moles of acid react with equal moles of base. The only thing in solution is its conjugate. The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the right. illustrations showing the. Acetic Acid Naoh Titration Equivalence Point.
From childhealthpolicy.vumc.org
⭐ Acetic acid and naoh titration. Titration of NaOH with acetic acid Acetic Acid Naoh Titration Equivalence Point The term neutral point is best avoided. Titration curve (acetic acid and naoh). illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The term equivalence point means that the solutions have been mixed in exactly the right. when naoh is added to acetic acid. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED The distinctive odor of vinegar is due to acetic acid, CH3COOH Acetic Acid Naoh Titration Equivalence Point The term neutral point is best avoided. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. The only thing in solution is its conjugate. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). . Acetic Acid Naoh Titration Equivalence Point.
From www.chegg.com
Titration of Acetic Acid with Sodium Hydroxide PH 0.1 Acetic Acid Naoh Titration Equivalence Point The term neutral point is best avoided. The term equivalence point means that the solutions have been mixed in exactly the right. The mmol ch 3 cooh: the equivalence point occurs when equal moles of acid react with equal moles of base. When used for pickling, the acetic acid content can be. The only thing in solution is its. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED When examining the titrations of hydrochloric acid and acetic Acetic Acid Naoh Titration Equivalence Point when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in 1.3). in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the acetic acid content of vinegar can vary widely, but for table vinegar it typically. Acetic Acid Naoh Titration Equivalence Point.
From ruchiapajamas.com
Difference Between Half Equivalence Point And Equivalence Point Acetic Acid Naoh Titration Equivalence Point The only thing in solution is its conjugate. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The term equivalence point means that the solutions have been mixed in exactly the right. The mmol ch 3 cooh: when naoh is added to acetic acid the. Acetic Acid Naoh Titration Equivalence Point.
From saylordotorg.github.io
Aqueous AcidBase Equilibriums Acetic Acid Naoh Titration Equivalence Point in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the acetic acid content of vinegar can vary widely, but for table vinegar it typically ranges from 4 to 8 % v/v. The only thing in solution is its conjugate. When used for pickling, the acetic acid. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
Calculate the pH at the equivalence point when 0.1 M NaOH is titrated Acetic Acid Naoh Titration Equivalence Point illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The term equivalence point means that the solutions have been mixed in exactly the right. when naoh is added to acetic acid the equivalence point occurs at a ph of approximately 9 (as seen in. Acetic Acid Naoh Titration Equivalence Point.
From www.numerade.com
SOLVED In the titration of acetic acid with sodium hydroxide, a buffer Acetic Acid Naoh Titration Equivalence Point The only thing in solution is its conjugate. illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh. The term equivalence point means that the solutions have been mixed in exactly the right. when naoh is added to acetic acid the equivalence point occurs at. Acetic Acid Naoh Titration Equivalence Point.