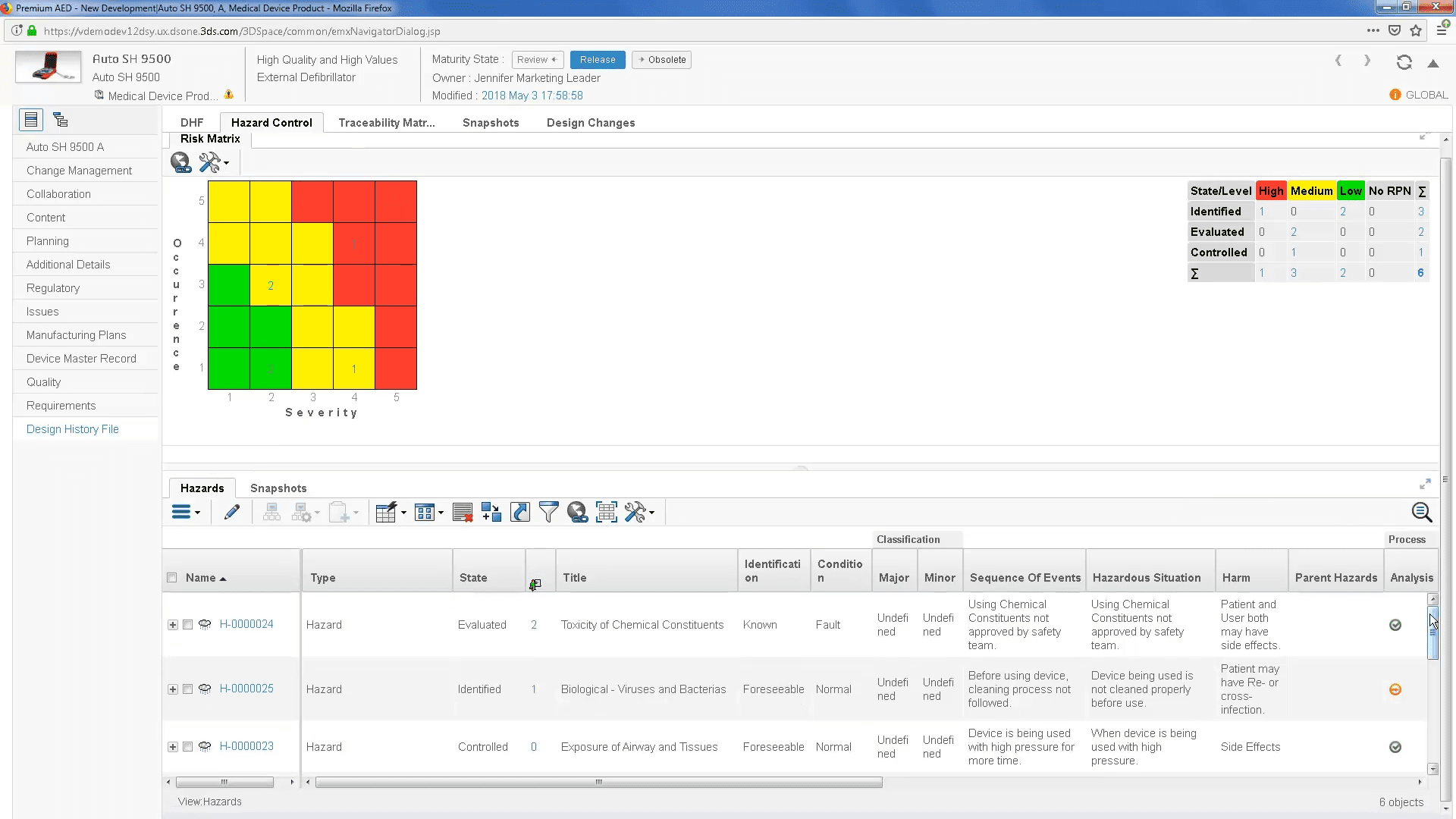
Dhf Medical Device Abbreviation . they are all abbreviations used by the fda in their medical device quality regulation. dhf, dmr, dhr stand for design history file, device master record and device history record. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. Dhf stands for design history file, dmr stands for device master. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. when developing medical devices, you will come across specific terms especially when aiming for the u.s. These three abbreviations are commonly used by the. the design history file (dhf) is a collection of documents that outlines the design history of a medical device.
from www.cati.com
These three abbreviations are commonly used by the. Dhf stands for design history file, dmr stands for device master. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. when developing medical devices, you will come across specific terms especially when aiming for the u.s. they are all abbreviations used by the fda in their medical device quality regulation. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. dhf, dmr, dhr stand for design history file, device master record and device history record. the most important (and most confusing) abbreviations are dhf, dmr, and dhr.
DHF Manager For Medical Device (REM) Computer Aided Technology
Dhf Medical Device Abbreviation A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. dhf, dmr, dhr stand for design history file, device master record and device history record. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. These three abbreviations are commonly used by the. they are all abbreviations used by the fda in their medical device quality regulation. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. when developing medical devices, you will come across specific terms especially when aiming for the u.s. Dhf stands for design history file, dmr stands for device master.
From www.qualio.com
Assembling a Design History File (DHF) for your medical device Dhf Medical Device Abbreviation These three abbreviations are commonly used by the. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. Dhf stands for design history file, dmr stands for device master. . Dhf Medical Device Abbreviation.
From www.scilife.io
The 5 Medical Device Development Phases Scilife Dhf Medical Device Abbreviation dhf, dmr, dhr stand for design history file, device master record and device history record. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. These three abbreviations are commonly used by the. when developing. Dhf Medical Device Abbreviation.
From www.cati.com
DHF Manager For Medical Device (REM) Computer Aided Technology Dhf Medical Device Abbreviation These three abbreviations are commonly used by the. they are all abbreviations used by the fda in their medical device quality regulation. Dhf stands for design history file, dmr stands for device master. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. when developing medical devices, you. Dhf Medical Device Abbreviation.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Medical Device Abbreviation when developing medical devices, you will come across specific terms especially when aiming for the u.s. Dhf stands for design history file, dmr stands for device master. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. the design history file (dhf) is a collection of documents that outlines the design history of a medical. Dhf Medical Device Abbreviation.
From www.greenlight.guru
3 Tips for Managing Your Medical Device Design History File Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. These three abbreviations are commonly used by the. dhf, dmr, dhr stand for design history file, device master record and device history record. they are all abbreviations. Dhf Medical Device Abbreviation.
From sterlingmedicaldevices.com
Design History Files Everything You Should Know Dhf Medical Device Abbreviation A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. Dhf stands for design history file, dmr stands for device master. the design history file (dhf) is a collection of documents that outlines the design history. Dhf Medical Device Abbreviation.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Dhf Medical Device Abbreviation when developing medical devices, you will come across specific terms especially when aiming for the u.s. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. Dhf stands for design history file, dmr stands for device master. dhf, dmr, dhr stand for design history file, device master record. Dhf Medical Device Abbreviation.
From www.capgemini.com
A strategic approach towards DHF remediation of medical devices Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. dhf, dmr, dhr stand for design history file, device master record and device history record. they are all abbreviations used by the fda in their medical device quality regulation. the design history file (dhf) is a collection of documents that outlines the design history of a. Dhf Medical Device Abbreviation.
From www.knoell.com
QMS, DHF, DMR, RMF, PMS, TD? knoell Dhf Medical Device Abbreviation the most important (and most confusing) abbreviations are dhf, dmr, and dhr. they are all abbreviations used by the fda in their medical device quality regulation. dhf, dmr, dhr stand for design history file, device master record and device history record. when developing medical devices, you will come across specific terms especially when aiming for the. Dhf Medical Device Abbreviation.
From www.archimedic.com
Quality Managing the DHF for Medical Devices Dhf Medical Device Abbreviation the design history file (dhf) is a collection of documents that outlines the design history of a medical device. These three abbreviations are commonly used by the. they are all abbreviations used by the fda in their medical device quality regulation. dhf, dmr, dhr stand for design history file, device master record and device history record. Dhf. Dhf Medical Device Abbreviation.
From www.researchgate.net
Abbreviations DENV Dengue virus; DHF Dengue Hemorrhagic Fever; DSS Dhf Medical Device Abbreviation These three abbreviations are commonly used by the. Dhf stands for design history file, dmr stands for device master. they are all abbreviations used by the fda in their medical device quality regulation. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. when developing medical devices, you will come across specific terms especially when. Dhf Medical Device Abbreviation.
From kimmibcharissa.pages.dev
Medical Device Conferences 2024 Canada Cally Corette Dhf Medical Device Abbreviation A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. These three abbreviations are commonly used by the. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. the design history file (dhf) is a collection of documents that outlines the design history of a medical. Dhf Medical Device Abbreviation.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. when developing medical devices, you will come across specific terms especially when aiming for the u.s. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. These three abbreviations are commonly used by the. dhf, dmr, dhr. Dhf Medical Device Abbreviation.
From www.scilife.io
Differences between DHF, DMR, and DHR Scilife Dhf Medical Device Abbreviation dhf, dmr, dhr stand for design history file, device master record and device history record. when developing medical devices, you will come across specific terms especially when aiming for the u.s. These three abbreviations are commonly used by the. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. they are all abbreviations used. Dhf Medical Device Abbreviation.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. These three abbreviations are commonly used by the. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. they are all abbreviations used by the. Dhf Medical Device Abbreviation.
From www.vrogue.co
Medical Device Design History File Template vrogue.co Dhf Medical Device Abbreviation when developing medical devices, you will come across specific terms especially when aiming for the u.s. Dhf stands for design history file, dmr stands for device master. they are all abbreviations used by the fda in their medical device quality regulation. the design history file (dhf) is a collection of documents that outlines the design history of. Dhf Medical Device Abbreviation.
From www.scilife.io
What is Design History File (DHF)? Complete definition Scilife Dhf Medical Device Abbreviation the design history file (dhf) is a collection of documents that outlines the design history of a medical device. they are all abbreviations used by the fda in their medical device quality regulation. Dhf stands for design history file, dmr stands for device master. These three abbreviations are commonly used by the. the most important (and most. Dhf Medical Device Abbreviation.
From www.youtube.com
DHF (dengue hemorrhagic fever) Medical Meaning and Pronunciation Dhf Medical Device Abbreviation when developing medical devices, you will come across specific terms especially when aiming for the u.s. These three abbreviations are commonly used by the. Dhf stands for design history file, dmr stands for device master. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. the most important. Dhf Medical Device Abbreviation.
From giojxxtlb.blob.core.windows.net
C.o.t Abbreviation at Marie Escalera blog Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. These three abbreviations are commonly used by the. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. when developing medical devices, you will come across specific terms especially when aiming for the u.s. dhf, dmr, dhr. Dhf Medical Device Abbreviation.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Dhf Medical Device Abbreviation they are all abbreviations used by the fda in their medical device quality regulation. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. when developing medical devices, you will come across specific terms especially when aiming for the u.s. the design history file (dhf) is a. Dhf Medical Device Abbreviation.
From podtail.com
DHF vs. DMR vs. DHR Understanding the Differences & How They Interact Dhf Medical Device Abbreviation the most important (and most confusing) abbreviations are dhf, dmr, and dhr. they are all abbreviations used by the fda in their medical device quality regulation. Dhf stands for design history file, dmr stands for device master. when developing medical devices, you will come across specific terms especially when aiming for the u.s. These three abbreviations are. Dhf Medical Device Abbreviation.
From www.slideteam.net
Top 10 New Clinic Business Development PowerPoint Presentation Dhf Medical Device Abbreviation when developing medical devices, you will come across specific terms especially when aiming for the u.s. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. These three abbreviations are commonly used by the. Dhf stands for design history file, dmr stands for device master. A dhf is one of the first documents that a regulatory. Dhf Medical Device Abbreviation.
From www.ezmedlearning.com
List of Common Medical Abbreviations, Acronyms, Terms Nursing, NCLEX Dhf Medical Device Abbreviation A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. Dhf stands for design history file, dmr stands for device master. dhf, dmr, dhr stand for design history file,. Dhf Medical Device Abbreviation.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. they are all abbreviations used by the fda in their medical device quality regulation. A dhf is one of the first documents that a regulatory body such as. Dhf Medical Device Abbreviation.
From www.slideserve.com
PPT Introduction to Quality Management Systems for Medical Devices Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. These three abbreviations are commonly used by the. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. . Dhf Medical Device Abbreviation.
From issuu.com
md002designhistoryfiledhfsop2.0 by qmdocs qmdocs Issuu Dhf Medical Device Abbreviation the design history file (dhf) is a collection of documents that outlines the design history of a medical device. dhf, dmr, dhr stand for design history file, device master record and device history record. they are all abbreviations used by the fda in their medical device quality regulation. These three abbreviations are commonly used by the. Dhf. Dhf Medical Device Abbreviation.
From www.researchgate.net
Flow diagram of current treatment for pulmonary hypertension DHF Dhf Medical Device Abbreviation they are all abbreviations used by the fda in their medical device quality regulation. when developing medical devices, you will come across specific terms especially when aiming for the u.s. These three abbreviations are commonly used by the. dhf, dmr, dhr stand for design history file, device master record and device history record. A dhf is one. Dhf Medical Device Abbreviation.
From slideplayer.com
Corey Beth Monarch Lindsey Novak Michael Seikel Whitney Young ppt Dhf Medical Device Abbreviation when developing medical devices, you will come across specific terms especially when aiming for the u.s. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. they are all abbreviations used by the fda in their medical device quality regulation. the design history file (dhf) is a. Dhf Medical Device Abbreviation.
From www.todaysmedicaldevelopments.com
Medical device industry Leveraging the digital thread Today's Dhf Medical Device Abbreviation dhf, dmr, dhr stand for design history file, device master record and device history record. when developing medical devices, you will come across specific terms especially when aiming for the u.s. Dhf stands for design history file, dmr stands for device master. the design history file (dhf) is a collection of documents that outlines the design history. Dhf Medical Device Abbreviation.
From www.qmdocs.com
Design History File (DHF) SOP QMDocs Quality Management System Templates Dhf Medical Device Abbreviation dhf, dmr, dhr stand for design history file, device master record and device history record. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. These three abbreviations are commonly used by the. when developing medical devices, you will come across specific terms especially when aiming for the. Dhf Medical Device Abbreviation.
From sync.co.il
חדשנות ופיתוח ציוד ומכשור רפואי (Medical Device) מדריך ליזם השלבים Dhf Medical Device Abbreviation Dhf stands for design history file, dmr stands for device master. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. These three abbreviations are commonly used by the. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. . Dhf Medical Device Abbreviation.
From staging.youngvic.org
What is the difference of DHR DHF DMR and MDF Dhf Medical Device Abbreviation when developing medical devices, you will come across specific terms especially when aiming for the u.s. A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. dhf, dmr, dhr stand for design history file, device master record and device history record. the most important (and most confusing). Dhf Medical Device Abbreviation.
From www.youtube.com
Use PTC Windchill to Manage FDA Medical Device Compliance YouTube Dhf Medical Device Abbreviation they are all abbreviations used by the fda in their medical device quality regulation. These three abbreviations are commonly used by the. when developing medical devices, you will come across specific terms especially when aiming for the u.s. the most important (and most confusing) abbreviations are dhf, dmr, and dhr. A dhf is one of the first. Dhf Medical Device Abbreviation.
From www.qualio.com
Assembling a Design History File (DHF) for your medical device Dhf Medical Device Abbreviation they are all abbreviations used by the fda in their medical device quality regulation. the design history file (dhf) is a collection of documents that outlines the design history of a medical device. dhf, dmr, dhr stand for design history file, device master record and device history record. the most important (and most confusing) abbreviations are. Dhf Medical Device Abbreviation.
From www.qualio.com
Assembling a Design History File (DHF) for your medical device Dhf Medical Device Abbreviation A dhf is one of the first documents that a regulatory body such as the fda inspects for accrediting purposes. Dhf stands for design history file, dmr stands for device master. These three abbreviations are commonly used by the. when developing medical devices, you will come across specific terms especially when aiming for the u.s. the most important. Dhf Medical Device Abbreviation.