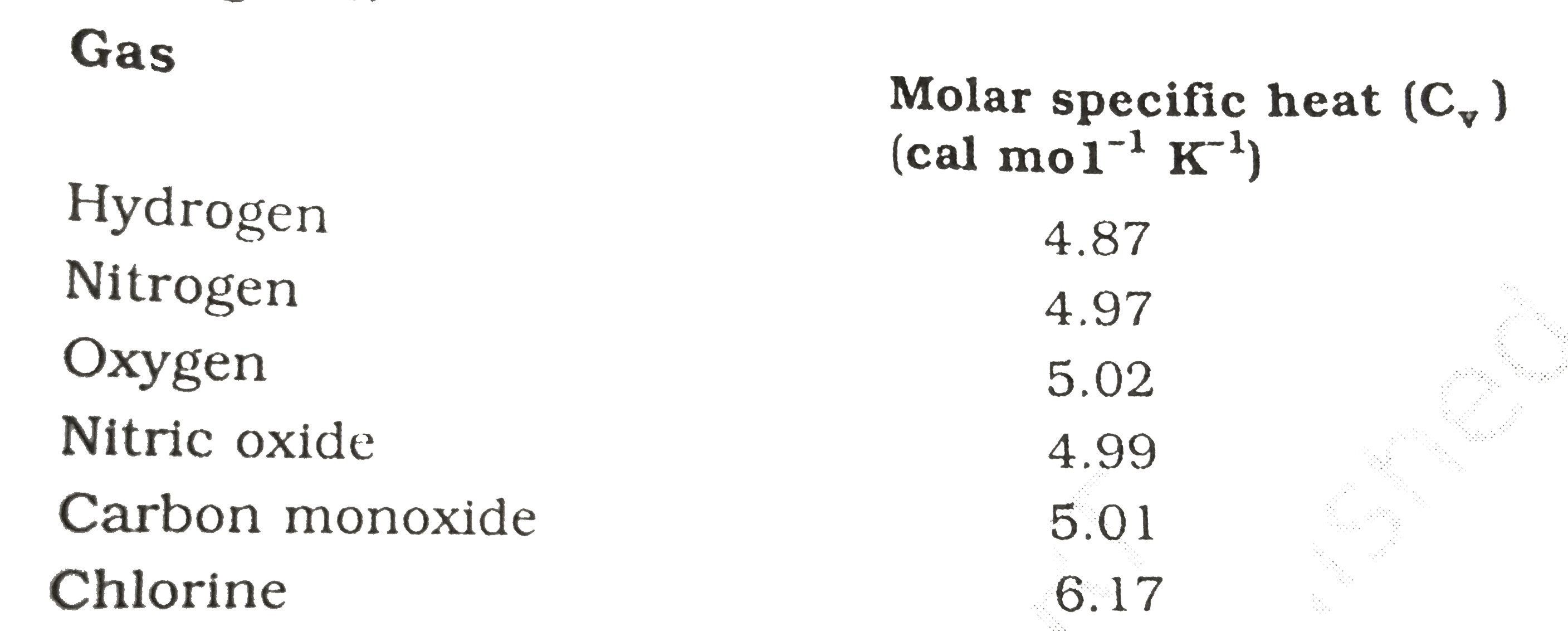Molar Specific Heat Capacity Of Nitrogen Gas Is . The molar specific heats of ideal monoatomic gases are: The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Molar specific heats of gases. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Specific heat of nitrogen is 1.04 j/g k. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. When a given amount of heat is added to different substances, their temperatures increase by different amounts. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g.
from www.doubtnut.com
S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. Specific heat of nitrogen is 1.04 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. The molar specific heats of ideal monoatomic gases are: When a given amount of heat is added to different substances, their temperatures increase by different amounts. Molar specific heats of gases. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and.
Given below are observations on molar specific heats at room temperatu
Molar Specific Heat Capacity Of Nitrogen Gas Is For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. When a given amount of heat is added to different substances, their temperatures increase by different amounts. Specific heat of nitrogen is 1.04 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. The molar specific heats of ideal monoatomic gases are: Molar specific heats of gases.
From www.bartleby.com
Molar Specific Heat bartleby Molar Specific Heat Capacity Of Nitrogen Gas Is C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. Molar specific heats of. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Heat Capacity Of Nitrogen Gas Molar Specific Heat Capacity Of Nitrogen Gas Is C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Molar specific heats of gases. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.bartleby.com
Molar Specific Heat bartleby Molar Specific Heat Capacity Of Nitrogen Gas Is 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat of nitrogen is 1.04 j/g k. When a given amount of heat is added to different substances, their temperatures increase by different amounts. Molar specific heats of gases. C p = heat capacity (j/mol*k). Molar Specific Heat Capacity Of Nitrogen Gas Is.
From eduinput.com
Molar Specific HeatSpecific heat, And Types Molar Specific Heat Capacity Of Nitrogen Gas Is Molar specific heats of gases. The molar specific heats of ideal monoatomic gases are: For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. S° = a*ln (t) + b*t + c*t 2. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.scribd.com
Molar Specific Heats of Other Materials PDF Heat Capacity Gases Molar Specific Heat Capacity Of Nitrogen Gas Is C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. Molar specific heats of gases. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. 55 rows the table of specific. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.chegg.com
Solved 9. The molar heat capacity of nitrogen at 1 bar is Molar Specific Heat Capacity Of Nitrogen Gas Is C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Molar specific heats of gases. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Specific heat of nitrogen is 1.04 j/g k. S° = a*ln (t) + b*t + c*t 2 /2 +. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.youtube.com
`C_(v)` denotes the molar specific heat of a gas at constant volume and `gamma YouTube Molar Specific Heat Capacity Of Nitrogen Gas Is The molar specific heats of ideal monoatomic gases are: When a given amount of heat is added to different substances, their temperatures increase by different amounts. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Molar specific heats of gases. For diatomic or linear polyatomic. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.youtube.com
Molar Specific Heats; C_v and C_p YouTube Molar Specific Heat Capacity Of Nitrogen Gas Is 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. Specific heat of nitrogen is 1.04 j/g k. The specific heat (= specific heat capacity) at. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.chegg.com
Solved Compute the specific heat capacity at constant volume Molar Specific Heat Capacity Of Nitrogen Gas Is Specific heat of nitrogen is 1.04 j/g k. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. When a given amount of heat is added to different substances, their temperatures increase by different amounts. The. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.slideserve.com
PPT Chapter 6 Principles of Reactivity Energy and Chemical Reactions Thermochemistry Molar Specific Heat Capacity Of Nitrogen Gas Is C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. When a given amount of heat is added to different substances, their temperatures increase by different amounts. 55 rows the table of specific heat. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.toppr.com
Cv and Cp denote the molar specific heat capacities of a gas at constant volume and constant Molar Specific Heat Capacity Of Nitrogen Gas Is Specific heat of nitrogen is 1.04 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The molar specific heats of ideal monoatomic gases are: For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. Molar specific heats of gases. When. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.toppr.com
For polytropic process {PV}^{n}= constant, {C}_{m} (molar heat capacity) of an ideal gas is Molar Specific Heat Capacity Of Nitrogen Gas Is When a given amount of heat is added to different substances, their temperatures increase by different amounts. Molar specific heats of gases. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.toppr.com
An ideal diatomic, gas undergoes a polytropic process described by the equation P√(V) constant Molar Specific Heat Capacity Of Nitrogen Gas Is The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. When a given amount of heat is added to different substances, their temperatures increase by. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.doubtnut.com
Given below are observations on molar specific heats at room temperatu Molar Specific Heat Capacity Of Nitrogen Gas Is For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. Specific heat of nitrogen is 1.04 j/g k. The molar specific heats of ideal monoatomic gases are: C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. When a given amount of heat is added to different substances, their temperatures increase by. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From byjus.com
ideal monoatomic gas is takebn through a process dQ=2dU.Find the molar heat capacity(in terms of Molar Specific Heat Capacity Of Nitrogen Gas Is S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Specific heat of nitrogen is 1.04 j/g k. 55 rows the table of specific heat capacities gives the volumetric. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From askfilo.com
The graph illustrates how the molar specific heat capacity at constant vo.. Molar Specific Heat Capacity Of Nitrogen Gas Is 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat of nitrogen is 1.04 j/g k. Molar specific heats of gases. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. C p = heat capacity (j/mol*k) h° = standard enthalpy. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.researchgate.net
Molar specific heat at constant volume vs. temperature for N2 (left... Download Scientific Diagram Molar Specific Heat Capacity Of Nitrogen Gas Is When a given amount of heat is added to different substances, their temperatures increase by different amounts. The molar specific heats of ideal monoatomic gases are: S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. The. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation ID588347 Molar Specific Heat Capacity Of Nitrogen Gas Is Molar specific heats of gases. The molar specific heats of ideal monoatomic gases are: When a given amount of heat is added to different substances, their temperatures increase by different amounts. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.youtube.com
Molar Heat Capacity Problems Physics YouTube Molar Specific Heat Capacity Of Nitrogen Gas Is The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. When a given amount of heat is added to different substances, their temperatures increase by different amounts. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.numerade.com
SOLVED Compute the specific heat capacity at constant volume of nitrogen (N2) gas. The molar Molar Specific Heat Capacity Of Nitrogen Gas Is S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. The molar specific heats of ideal monoatomic gases are: When a given amount of heat is added to different substances, their temperatures increase by different amounts. Specific heat of nitrogen is 1.04 j/g k. C p = heat capacity (j/mol*k). Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free download ID4829142 Molar Specific Heat Capacity Of Nitrogen Gas Is The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Molar specific heats of gases. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. The molar specific heats of ideal. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.toppr.com
Molar specific heat capacity of a gas can be negative, positive or zero. Molar Specific Heat Capacity Of Nitrogen Gas Is 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. When a. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.toppr.com
The Molar heat capacities of nitrogen at constant pressure and constant volume are 29.11 kJ/k Molar Specific Heat Capacity Of Nitrogen Gas Is The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Molar specific heats of gases. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. When a given amount of heat is added to different substances, their temperatures increase by different amounts. 55 rows. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.chegg.com
Solved Compute the specific heat capacity at constant volume Molar Specific Heat Capacity Of Nitrogen Gas Is Specific heat of nitrogen is 1.04 j/g k. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. When a given amount of heat is added to different substances,. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.numerade.com
SOLVED Assume the constant pressure heat capacity of nitrogen gas is 30.000 K1 mol1 at 300 K Molar Specific Heat Capacity Of Nitrogen Gas Is The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Molar specific heats of gases. The molar specific heats of ideal monoatomic gases are: S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. When a given amount of. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.chegg.com
Solved Part A Compute the specific heat capacity at constant Molar Specific Heat Capacity Of Nitrogen Gas Is Specific heat of nitrogen is 1.04 j/g k. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2). Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation ID588347 Molar Specific Heat Capacity Of Nitrogen Gas Is Specific heat of nitrogen is 1.04 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. When a given amount of heat is added to different substances, their temperatures increase by different amounts. S° = a*ln (t) + b*t + c*t 2 /2 +. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From ar.inspiredpencil.com
Heat Capacity Chart Molar Specific Heat Capacity Of Nitrogen Gas Is The molar specific heats of ideal monoatomic gases are: S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Molar specific heats of gases. When a given amount of heat is added to different substances,. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.youtube.com
Molar Specific Heat of solids, liquids and gases. Dulong Petit's law. Thermal Heat Capacity Molar Specific Heat Capacity Of Nitrogen Gas Is Specific heat of nitrogen is 1.04 j/g k. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. The molar specific heats of ideal monoatomic gases are: The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. 55 rows the table of specific heat. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.youtube.com
specific heat capacity /molar specific heat capacity of solids/ C= 3R derivation Molar Specific Heat Capacity Of Nitrogen Gas Is C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. Specific heat of nitrogen is 1.04 j/g k. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. When a given amount of heat is added to different substances, their temperatures increase by different. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.slideserve.com
PPT ENG2000 Chapter 9 Thermal Properties of Materials PowerPoint Presentation ID6760036 Molar Specific Heat Capacity Of Nitrogen Gas Is For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Specific heat of nitrogen is 1.04 j/g k. Molar specific heats of gases. The molar specific heats of ideal monoatomic gases are: 55 rows the table. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.bartleby.com
Molar Specific Heat bartleby Molar Specific Heat Capacity Of Nitrogen Gas Is Molar specific heats of gases. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. Specific heat of nitrogen is 1.04 j/g. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity Molar Specific Heat Capacity Of Nitrogen Gas Is For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. The specific heat (= specific heat capacity) at constant pressure and constant. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.numerade.com
SOLVED A) Compute the specific heat capacity at constant volume of nitrogen (N2) gas. The molar Molar Specific Heat Capacity Of Nitrogen Gas Is 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat of nitrogen is 1.04 j/g k. For diatomic or linear polyatomic molecules, two rotational degrees of freedom are added,. C p = heat capacity (j/mol*k) h° = standard enthalpy (kj/mol) s° = standard entropy.. Molar Specific Heat Capacity Of Nitrogen Gas Is.
From www.chegg.com
Solved Part A Compute the specific heat capacity at constant Molar Specific Heat Capacity Of Nitrogen Gas Is The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Molar specific heats of gases. S° = a*ln (t) + b*t + c*t 2 /2 + d*t 3 /3 − e/ (2*t 2) + g. 55 rows the table of specific heat capacities gives the volumetric heat capacity as. Molar Specific Heat Capacity Of Nitrogen Gas Is.