What Is A Buffer How Does It Work . Each buffer is characterized by a set. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. It is able to neutralize small. Either a weak acid plus a salt derived from that. If we mix a weak acid (ha) with its conjugate base (a. Buffers do so by being composed of certain pairs of solutes:
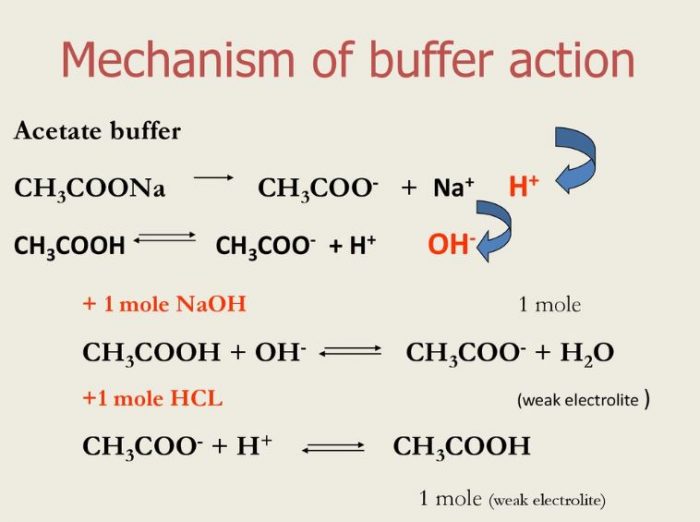
from classnotes.org.in
A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. Either a weak acid plus a salt derived from that. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? The mechanism involves a buffer, a solution that resists dramatic changes in ph. If we mix a weak acid (ha) with its conjugate base (a. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Each buffer is characterized by a set. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature.
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium
What Is A Buffer How Does It Work If we mix a weak acid (ha) with its conjugate base (a. It is able to neutralize small. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Each buffer is characterized by a set. If we mix a weak acid (ha) with its conjugate base (a. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small.
From www.brainkart.com
How do buffers work? What Is A Buffer How Does It Work Buffers do so by being composed of certain pairs of solutes: Each buffer is characterized by a set. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a. What Is A Buffer How Does It Work.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk What Is A Buffer How Does It Work Buffers do so by being composed of certain pairs of solutes: A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? Each buffer is characterized by. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download ID5348308 What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. If we mix a weak acid (ha) with its conjugate. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT 18 Additional Aspects of AcidBase Equilibria PowerPoint Presentation ID2832 What Is A Buffer How Does It Work Each buffer is characterized by a set. It is able to neutralize small. Buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition. What Is A Buffer How Does It Work.
From www.youtube.com
How do buffers work, paper 1 AQA A level Chemistry YouTube What Is A Buffer How Does It Work It is able to neutralize small. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes?. What Is A Buffer How Does It Work.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer How Does It Work Either a weak acid plus a salt derived from that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Each buffer is characterized by a set. A buffer. What Is A Buffer How Does It Work.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital Circuits ? YouTube What Is A Buffer How Does It Work It is able to neutralize small. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is. What Is A Buffer How Does It Work.
From wirelistetiquette.z13.web.core.windows.net
How Does A Buffer Tank Work What Is A Buffer How Does It Work It is able to neutralize small. Either a weak acid plus a salt derived from that. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. Each buffer is characterized by a set. A buffer is an aqueous solution that can resist. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5845696 What Is A Buffer How Does It Work It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. How does a. What Is A Buffer How Does It Work.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer How Does It Work It is able to neutralize small. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. It is able to neutralize small. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph. What Is A Buffer How Does It Work.
From www.slideshare.net
Buffer system What Is A Buffer How Does It Work Each buffer is characterized by a set. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. A buffer is a solution that can resist ph change upon the addition of. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Is A Buffer How Does It Work A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. It is able to neutralize small. Each buffer is. What Is A Buffer How Does It Work.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer How Does It Work How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? If we mix a weak acid (ha) with its conjugate base (a. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is a solution where. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Is A Buffer How Does It Work A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Either a weak acid plus a salt derived from that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID5083921 What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. Either a weak acid plus a salt derived from that. The mechanism involves. What Is A Buffer How Does It Work.
From www.youtube.com
How Do Buffers Work? With Buffer Calculations! AP Chemistry Unit 8.3 YouTube What Is A Buffer How Does It Work A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? If we mix a weak acid (ha) with its conjugate base (a. It is able to. What Is A Buffer How Does It Work.
From www.thoughtco.com
What Is a Buffer and How Does It Work? What Is A Buffer How Does It Work Buffers do so by being composed of certain pairs of solutes: A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. If we mix a weak acid (ha) with its conjugate base (a. A buffer solution is a solution where the ph does not change. What Is A Buffer How Does It Work.
From www.numerade.com
SOLVEDWhat is a buffer? How does a buffer work? How does it neutralize added acid? Added base? What Is A Buffer How Does It Work It is able to neutralize small. It is able to neutralize small. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Each buffer is. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT All About Buffers PowerPoint Presentation, free download ID1321295 What Is A Buffer How Does It Work It is able to neutralize small. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? Either a weak acid plus a salt derived from that. It is able to neutralize small. Buffers do so by being composed of certain pairs of solutes: The mechanism involves a buffer, a solution that. What Is A Buffer How Does It Work.
From 2012books.lardbucket.org
Buffers What Is A Buffer How Does It Work Either a weak acid plus a salt derived from that. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level. What Is A Buffer How Does It Work.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffers do so by being composed of certain pairs of solutes: A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download ID1072204 What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. A buffer is. What Is A Buffer How Does It Work.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk What Is A Buffer How Does It Work It is able to neutralize small. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? If we mix a weak acid (ha) with its conjugate. What Is A Buffer How Does It Work.
From www.youtube.com
More About Buffers (A2 Chemistry) YouTube What Is A Buffer How Does It Work The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? It is able to neutralize small. A buffer is. What Is A Buffer How Does It Work.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is A Buffer How Does It Work Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. It is able to. What Is A Buffer How Does It Work.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Is A Buffer How Does It Work It is able to neutralize small. It is able to neutralize small. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is a solution where the ph does. What Is A Buffer How Does It Work.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its action. YouTube What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. What Is A Buffer How Does It Work.
From www.tffn.net
How Does a Buffer Work? An InDepth Guide to Buffers and Their Chemistry The Enlightened Mindset What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If we mix a weak acid (ha) with its conjugate base (a. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. It. What Is A Buffer How Does It Work.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is A Buffer How Does It Work How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? The mechanism involves a buffer, a solution that resists dramatic changes in ph. If we mix a weak acid (ha) with its conjugate base (a. Either a weak acid plus a salt derived from that. It is able to neutralize small.. What Is A Buffer How Does It Work.
From www.slideshare.net
Buffer system What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. It is able to neutralize small. How does a mixture. What Is A Buffer How Does It Work.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is A Buffer How Does It Work Buffers do so by being composed of certain pairs of solutes: Each buffer is characterized by a set. It is able to neutralize small. How does a mixture of a weak acid and its conjugate base help buffer a solution against ph changes? A buffer solution is a solution where the ph does not change significantly on dilution or if. What Is A Buffer How Does It Work.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important To Homeostasis What Is A Buffer How Does It Work The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is an aqueous solution that can resist significant changes in ph levels upon the addition of a small amount of acid or alkali. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. What Is A Buffer How Does It Work.
From www.tffn.net
How Does a Buffer Work? An InDepth Guide to Buffers and Their Chemistry The Enlightened Mindset What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. What Is A Buffer How Does It Work.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is A Buffer How Does It Work If we mix a weak acid (ha) with its conjugate base (a. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Is A Buffer How Does It Work.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Is A Buffer How Does It Work A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is a solution where the ph. What Is A Buffer How Does It Work.