Ice Table Buffer Solution . solving for the ph of a prepared buffer solution using an ice box (table). ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. what is a buffer? Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. we can calculate the ph of a buffer using an ice chart! a buffer is created in a solution which contains both a weak acid and its conjugate base. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. let's say you are asked to calculate the ph of a solution with: Examples of ph calculations for buffer solutions.
from www.chegg.com
example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. let's say you are asked to calculate the ph of a solution with: solving for the ph of a prepared buffer solution using an ice box (table). ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. Examples of ph calculations for buffer solutions. what is a buffer? Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. a buffer is created in a solution which contains both a weak acid and its conjugate base. we can calculate the ph of a buffer using an ice chart!
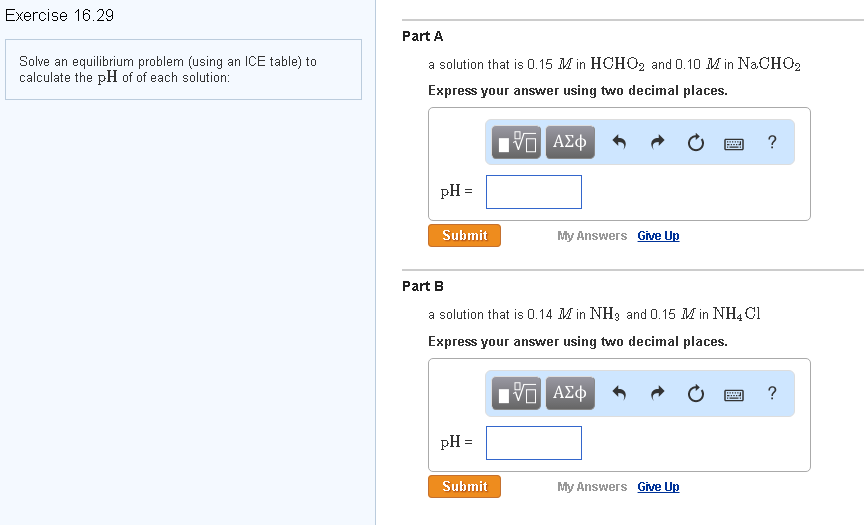
Solved Solve an equilibrium problem (using an ICE table) to
Ice Table Buffer Solution what is a buffer? example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. what is a buffer? Examples of ph calculations for buffer solutions. ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. let's say you are asked to calculate the ph of a solution with: a buffer is created in a solution which contains both a weak acid and its conjugate base. solving for the ph of a prepared buffer solution using an ice box (table). we can calculate the ph of a buffer using an ice chart!
From www.numerade.com
SOLVEDSolve an equilibrium problem (using an ICE table) to calculate Ice Table Buffer Solution ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. let's say you are asked to calculate the ph of a solution with: ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. solving for the ph of. Ice Table Buffer Solution.
From www.showme.com
ShowMe Ice table Ice Table Buffer Solution what is a buffer? example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. we can calculate the ph of a buffer using an ice chart! a buffer is created in. Ice Table Buffer Solution.
From www.chegg.com
Solved Determine the pH of a buffer solution by constructing Ice Table Buffer Solution Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. solving for the ph of a prepared buffer solution using an ice box (table). we can calculate the ph of a buffer using an ice chart! . Ice Table Buffer Solution.
From dxoniepxb.blob.core.windows.net
Ice Table In Chemistry at Terry Cordell blog Ice Table Buffer Solution what is a buffer? Examples of ph calculations for buffer solutions. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. let's say you are asked to calculate the ph of a solution with: we can calculate the ph of a buffer using an ice chart! ice (initial, change, equilibrium) tables. Ice Table Buffer Solution.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Ice Table Buffer Solution example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. Examples of ph calculations for buffer solutions. solving for the ph of a prepared buffer solution using an ice box (table). Buffers, or. Ice Table Buffer Solution.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Ice Table Buffer Solution let's say you are asked to calculate the ph of a solution with: ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. solving for the ph of a prepared buffer solution using an ice box (table). ice (initial, change, equilibrium) tables are very helpful tools for. Ice Table Buffer Solution.
From www.youtube.com
Equilibrium Reaction with an ICE Table Chemistry Sample Problem YouTube Ice Table Buffer Solution solving for the ph of a prepared buffer solution using an ice box (table). we can calculate the ph of a buffer using an ice chart! Examples of ph calculations for buffer solutions. a buffer is created in a solution which contains both a weak acid and its conjugate base. ice (initial, change, equilibrium) tables are. Ice Table Buffer Solution.
From www.youtube.com
Ksp Molar Solubility, Ice Tables, & Common Ion Effect YouTube Ice Table Buffer Solution what is a buffer? let's say you are asked to calculate the ph of a solution with: ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. solving for the ph of a prepared buffer solution using an ice box (table). a buffer is created in. Ice Table Buffer Solution.
From www.studypool.com
SOLUTION Calculating the ph of a buffer solution Studypool Ice Table Buffer Solution a buffer is created in a solution which contains both a weak acid and its conjugate base. solving for the ph of a prepared buffer solution using an ice box (table). what is a buffer? let's say you are asked to calculate the ph of a solution with: ice tables are composed of the concentrations. Ice Table Buffer Solution.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Ice Table Buffer Solution ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. solving for the ph of a prepared buffer solution using an ice box (table). example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. what is a buffer? let's say you. Ice Table Buffer Solution.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses Ice Table Buffer Solution ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. we can calculate the ph of a buffer using an ice chart! a buffer is created in a solution which contains both a weak acid and its conjugate base. example of calculating the ph of solution that. Ice Table Buffer Solution.
From www.youtube.com
Buffer calculation (partial neutralisation) using ICE method YouTube Ice Table Buffer Solution what is a buffer? ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. example of calculating the ph of solution that is 1.00 m acetic acid and. Ice Table Buffer Solution.
From www.slideshare.net
CM4106 Review of Lesson 3 (Part 1) Ice Table Buffer Solution we can calculate the ph of a buffer using an ice chart! what is a buffer? ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. Buffers, or. Ice Table Buffer Solution.
From www.chegg.com
Solved Determine the concentration of C6H5NH3+in a buffer Ice Table Buffer Solution we can calculate the ph of a buffer using an ice chart! Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. a buffer is created in a solution which contains both a weak acid and its conjugate base. ice tables are composed of the concentrations of molecules in solution in different. Ice Table Buffer Solution.
From www.youtube.com
Using an ICE table to solve for Kc YouTube Ice Table Buffer Solution ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. a buffer is created in a solution which contains both a weak acid and its conjugate base. let's say you are asked to calculate the ph of a solution with: what is a buffer? ice (initial,. Ice Table Buffer Solution.
From www.mcguffmedical.com
pH Calibrating Buffer Solution, pH 4.01, 475mL, Each McGuff Medical Ice Table Buffer Solution Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. what is a buffer? a buffer is created in a solution which contains both a weak acid and its conjugate base. solving. Ice Table Buffer Solution.
From www.youtube.com
Week 9 9. pH of a buffer using an ICE chart YouTube Ice Table Buffer Solution Examples of ph calculations for buffer solutions. we can calculate the ph of a buffer using an ice chart! let's say you are asked to calculate the ph of a solution with: ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. ice tables are composed of. Ice Table Buffer Solution.
From www.youtube.com
Finding pH of a buffer system using ICE table YouTube Ice Table Buffer Solution solving for the ph of a prepared buffer solution using an ice box (table). what is a buffer? example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. Examples of ph calculations. Ice Table Buffer Solution.
From studylib.net
Buffer Solutions Ice Table Buffer Solution ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. what is a buffer? a buffer is created in a solution which contains both a weak acid and its conjugate base. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. Buffers,. Ice Table Buffer Solution.
From www.youtube.com
Quadratic Equation ICE Table Equilibrium Calculations YouTube Ice Table Buffer Solution a buffer is created in a solution which contains both a weak acid and its conjugate base. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. we can calculate the ph. Ice Table Buffer Solution.
From www.chegg.com
(Buffers) Fill the following tables using the ICE Ice Table Buffer Solution a buffer is created in a solution which contains both a weak acid and its conjugate base. let's say you are asked to calculate the ph of a solution with: example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice (initial, change, equilibrium) tables are very helpful tools for understanding. Ice Table Buffer Solution.
From chemistryguru.com.sg
Determine pH of Resultant Solution of AcidBase Reaction Ice Table Buffer Solution ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. let's say you are asked to calculate the ph of a solution with: we can calculate the ph of a buffer using an ice chart! Buffers, or buffered solutions, resist changes in ph upon the addition of acid. Ice Table Buffer Solution.
From general.chemistrysteps.com
The Common Ion Effect Chemistry Steps Ice Table Buffer Solution solving for the ph of a prepared buffer solution using an ice box (table). example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. what is a buffer? ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. we can calculate. Ice Table Buffer Solution.
From www.chegg.com
Solved 6. From the buffer table below Circle the buffer Ice Table Buffer Solution what is a buffer? ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. we can calculate. Ice Table Buffer Solution.
From www.chegg.com
Solved Determine the pH of a buffer solution by constructing Ice Table Buffer Solution a buffer is created in a solution which contains both a weak acid and its conjugate base. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. we can calculate the ph of a buffer using an ice chart! let's say you are asked to calculate the ph of a solution with:. Ice Table Buffer Solution.
From www.aakash.ac.in
Acidic Buffer Solution Definition, Calculation & Applications Ice Table Buffer Solution a buffer is created in a solution which contains both a weak acid and its conjugate base. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. Examples of ph calculations for buffer solutions. solving for the ph of a prepared buffer solution using an ice box (table). what is a. Ice Table Buffer Solution.
From www.chegg.com
Solved Solve an equilibrium problem (using an ICE table) to Ice Table Buffer Solution Examples of ph calculations for buffer solutions. we can calculate the ph of a buffer using an ice chart! a buffer is created in a solution which contains both a weak acid and its conjugate base. what is a buffer? example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. . Ice Table Buffer Solution.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Ice Table Buffer Solution let's say you are asked to calculate the ph of a solution with: ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. what is a buffer? example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. ice (initial, change, equilibrium). Ice Table Buffer Solution.
From slidetodoc.com
Lecture 3 Buffer and Isotonic solutions Lecture 4 Ice Table Buffer Solution example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. Examples of ph calculations for buffer solutions. let's say you are asked to calculate the ph of a solution with: what is a buffer? ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph. Ice Table Buffer Solution.
From www.youtube.com
Biochemistry Buffers ICE Problem With HendersonHasselbalch Ice Table Buffer Solution Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. let's say you are asked to calculate the ph of a solution with: solving for the ph of a prepared buffer solution using. Ice Table Buffer Solution.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog Ice Table Buffer Solution ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. solving for the ph of a prepared buffer solution using an ice box (table). example of calculating the. Ice Table Buffer Solution.
From www.chegg.com
Solved Complete the ICE table below for the addition of 1.00 Ice Table Buffer Solution Examples of ph calculations for buffer solutions. ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. what is a buffer? ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. solving for the ph of a prepared. Ice Table Buffer Solution.
From www.toppr.com
Buffer Solutions Definition, Types, Preparation, Examples and Videos Ice Table Buffer Solution ice (initial, change, equilibrium) tables are very helpful tools for understanding equilibrium and for calculating the ph of a. solving for the ph of a prepared buffer solution using an ice box (table). ice tables are composed of the concentrations of molecules in solution in different stages of a reaction, and are. Buffers, or buffered solutions, resist. Ice Table Buffer Solution.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Ice Table Buffer Solution a buffer is created in a solution which contains both a weak acid and its conjugate base. example of calculating the ph of solution that is 1.00 m acetic acid and 1.00. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. Examples of ph calculations for buffer solutions. let's say you. Ice Table Buffer Solution.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Ice Table Buffer Solution we can calculate the ph of a buffer using an ice chart! solving for the ph of a prepared buffer solution using an ice box (table). Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. ice tables are composed of the concentrations of molecules in solution in different stages of a. Ice Table Buffer Solution.