Why Dilution Is Important . Often, a worker will need to change the concentration of a solution by changing the amount. During titration, solutions are diluted so there is a larger volume of solution. Dilution of solutions helps us in many ways. Often, a worker will need to change the concentration of a solution by. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. why dilution is important? learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to dilute and concentrate solutions.
from www.slideserve.com
learn how to dilute and concentrate solutions. why dilution is important? to dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by changing the amount. Of course, the resulting solution is. learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration.
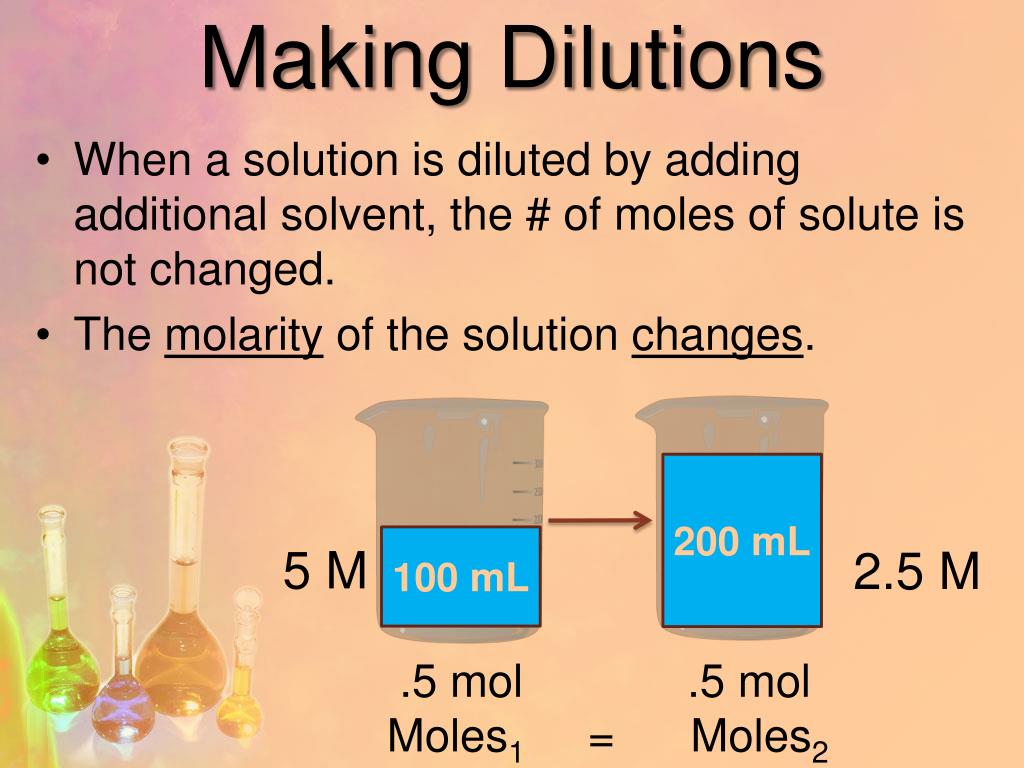
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free
Why Dilution Is Important learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which. During titration, solutions are diluted so there is a larger volume of solution. why dilution is important? a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by changing the amount. learn how to dilute and concentrate solutions. Dilution of solutions helps us in many ways. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration.
From carlosgokeowen.blogspot.com
What is Dilution Why Dilution Is Important learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. why dilution is important? Concentration is the removal of solvent, which. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. to dilute a solution means to. Why Dilution Is Important.
From www.numerade.com
SOLVED What is the key point to remember in serial dilution and Why Dilution Is Important in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. Concentration is the removal of solvent, which. Often, a worker will need to change the concentration of a solution by. to dilute a solution means to add more solvent without. Why Dilution Is Important.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Why Dilution Is Important Dilution of solutions helps us in many ways. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the amount. to dilute a solution means to add more solvent without the addition of more solute.. Why Dilution Is Important.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Why Dilution Is Important learn how to dilute and concentrate solutions. to dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which. Of course, the resulting solution is. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilute solution is one. Why Dilution Is Important.
From pressurecity.com
Chemical dilution chart Pressure City Why Dilution Is Important Of course, the resulting solution is. Dilution of solutions helps us in many ways. to dilute a solution means to add more solvent without the addition of more solute. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. in order to make a. Why Dilution Is Important.
From www.numerade.com
SOLVED Serial dilution is a common technique used in chemical analysis Why Dilution Is Important a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the amount. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to dilute and concentrate. Why Dilution Is Important.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog Why Dilution Is Important During titration, solutions are diluted so there is a larger volume of solution. why dilution is important? in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. a dilute solution is one in which there is a relatively small. Why Dilution Is Important.
From ar.inspiredpencil.com
Dilute Science Why Dilution Is Important Often, a worker will need to change the concentration of a solution by changing the amount. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. During titration, solutions are diluted so there is a larger volume of solution. Dilution of. Why Dilution Is Important.
From delune.co.uk
Why Diluting Essential Oils is Important Top 5 Reasons — Delune Why Dilution Is Important During titration, solutions are diluted so there is a larger volume of solution. Of course, the resulting solution is. learn how to dilute and concentrate solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the addition of solvent, which decreases the concentration of the. Why Dilution Is Important.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free Why Dilution Is Important learn how to dilute and concentrate solutions. During titration, solutions are diluted so there is a larger volume of solution. Often, a worker will need to change the concentration of a solution by changing the amount. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to. Why Dilution Is Important.
From www.youtube.com
Ionisation of weak electrolytes and Ostwalds dilution law YouTube Why Dilution Is Important why dilution is important? learn how to dilute and concentrate solutions. to dilute a solution means to add more solvent without the addition of more solute. Dilution of solutions helps us in many ways. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the. Why Dilution Is Important.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Why Dilution Is Important Of course, the resulting solution is. Often, a worker will need to change the concentration of a solution by changing the amount. Concentration is the removal of solvent, which. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. to. Why Dilution Is Important.
From www.slideserve.com
PPT WHY??? PowerPoint Presentation, free download ID1830717 Why Dilution Is Important in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. learn how to dilute and concentrate. Why Dilution Is Important.
From dxobyjbye.blob.core.windows.net
How To Make Dilutions In Lab at Drew Ballard blog Why Dilution Is Important dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the amount. During titration, solutions are diluted so there is a larger volume of solution. in order to make a solution, we often take a sample of a. Why Dilution Is Important.
From www.wfmed.com
Why is Diluting so Important? (Dilution Ratios included!) WFMED Why Dilution Is Important Often, a worker will need to change the concentration of a solution by changing the amount. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. to dilute a solution means to. Why Dilution Is Important.
From facts.net
13 Surprising Facts About Dilution Why Dilution Is Important Dilution of solutions helps us in many ways. Often, a worker will need to change the concentration of a solution by. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the amount. Concentration is the. Why Dilution Is Important.
From www.medicine.mcgill.ca
Serial Dilutions Why Dilution Is Important in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. Often, a worker will need to change the concentration of a solution by. why dilution is important? dilution is the addition of solvent, which decreases the concentration of the. Why Dilution Is Important.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Why Dilution Is Important learn how to dilute and concentrate solutions. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. During titration, solutions are diluted so there is a larger volume of solution. Often, a worker will need to change the concentration of a solution by. why dilution is important? in order to. Why Dilution Is Important.
From simplyearth.com
Simply Earth Dilution Chart Why Dilution Is Important Often, a worker will need to change the concentration of a solution by. Of course, the resulting solution is. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount. why dilution is important? in order to make a solution, we often take a sample. Why Dilution Is Important.
From www.pinterest.com
Why is Essential Oil Dilution Important? Robert Tisserand Essential Why Dilution Is Important dilution is the addition of solvent, which decreases the concentration of the solute in the solution. learn how to dilute and concentrate solutions. why dilution is important? in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. . Why Dilution Is Important.
From mage02.technogym.com
Printable Essential Oil Dilution Chart Why Dilution Is Important to dilute a solution means to add more solvent without the addition of more solute. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. learn how. Why Dilution Is Important.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID9598576 Why Dilution Is Important Of course, the resulting solution is. why dilution is important? Dilution of solutions helps us in many ways. learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to. Why Dilution Is Important.
From www.complete.so
Stock Dilution what is it and why does it matter? EDUCATION Why Dilution Is Important dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is. learn how to dilute and concentrate solutions. During titration, solutions are diluted so there is a larger volume of solution. a dilute solution is one in which there is a relatively small amount of solute. Why Dilution Is Important.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Why Dilution Is Important Often, a worker will need to change the concentration of a solution by. Often, a worker will need to change the concentration of a solution by changing the amount. why dilution is important? learn how to dilute and concentrate solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in. Why Dilution Is Important.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Why Dilution Is Important dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. learn how to dilute and concentrate solutions. Dilution of solutions helps us in many ways. Often, a worker will need to change the concentration of a solution by changing the amount. in order to. Why Dilution Is Important.
From www.pinterest.com
Ultimate Guide To Diluting Essential Oils Ratios, Charts & More Why Dilution Is Important in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. why dilution is important? dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration. Why Dilution Is Important.
From www.pinterest.com
Dilution is very important. Make sure you dilute your essential oils Why Dilution Is Important Concentration is the removal of solvent, which. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. learn how to dilute and concentrate solutions. Of course, the resulting solution is. Often, a worker will need to change the concentration of. Why Dilution Is Important.
From www.expressmicroscience.co.uk
Why are dilutions important? Express Micro Science Why Dilution Is Important learn how to dilute and concentrate solutions. During titration, solutions are diluted so there is a larger volume of solution. Concentration is the removal of solvent, which. Dilution of solutions helps us in many ways. why dilution is important? learn how to dilute and concentrate solutions. to dilute a solution means to add more solvent without. Why Dilution Is Important.
From www.youtube.com
How To Dilute Chemicals Dilution Ratios Explained! YouTube Why Dilution Is Important Of course, the resulting solution is. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. to dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by. During titration, solutions are diluted so there is. Why Dilution Is Important.
From dxoosyyqw.blob.core.windows.net
How To Dilute Essential Oils For Body Spray at Nathaniel Blessing blog Why Dilution Is Important dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Of course, the resulting solution is. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. to dilute a solution means to add. Why Dilution Is Important.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Why Dilution Is Important Often, a worker will need to change the concentration of a solution by changing the amount. Of course, the resulting solution is. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution of solutions helps us in many ways. in order to make a solution, we often take a sample of. Why Dilution Is Important.
From www.differencebetween.com
Difference Between Dilution and Concentration Compare the Difference Why Dilution Is Important Often, a worker will need to change the concentration of a solution by changing the amount. learn how to dilute and concentrate solutions. Of course, the resulting solution is. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Concentration is the removal of solvent, which. dilution is. Why Dilution Is Important.
From www.youtube.com
150 dilution.why need this dilution?2 easy methods to preparation Why Dilution Is Important During titration, solutions are diluted so there is a larger volume of solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of. Why Dilution Is Important.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Why Dilution Is Important learn how to dilute and concentrate solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. in order to make a solution, we often take a sample of a concentrated stock solution and add solvent to it to dilute it to the desired concentration. Dilution of solutions. Why Dilution Is Important.
From www.youtube.com
While diluting an acid why is it that the acid should be Why Dilution Is Important a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution of solutions helps us in many ways. Concentration is the removal of solvent, which. why dilution is important? Of course, the resulting solution is. dilution is the addition of solvent, which decreases the concentration of the solute. Why Dilution Is Important.