Beer's Law Lab Chegg . beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's law is only valid at the. Select the best statement about beer's law. There are two contributions to this fundamental. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the beer's law plot to determine the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution.
from www.chegg.com
beer’s law is a limiting law that is valid only for low concentrations of analyte. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. There are two contributions to this fundamental. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. Use the beer's law plot to determine the. Beer's law is only valid at the. Select the best statement about beer's law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its.
Solved Simulation Data and Report Submission Beer's Law
Beer's Law Lab Chegg Beer's law is only valid at the. beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Select the best statement about beer's law. There are two contributions to this fundamental. Use the beer's law plot to determine the. Beer's law is only valid at the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law is a limiting law that is valid only for low concentrations of analyte.
From www.chegg.com
Solved PreLab Assignment Beer's Law This experiment covers Beer's Law Lab Chegg Use the beer's law plot to determine the. Beer's law is only valid at the. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. in spectroscopy, beer’s law states that the absorption of light. Beer's Law Lab Chegg.
From www.chegg.com
Solved Name Experiment 6 Beer's Law Pre Lab Questions 1. Beer's Law Lab Chegg in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. There are two contributions to this fundamental. Beer's law is only valid at the. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). since the concentration, path length and. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law Name Date PRELAB QUESTIONS 1. What is the Beer's Law Lab Chegg in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer's law is only valid at the. There are two contributions to this fundamental. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing. Beer's Law Lab Chegg.
From www.chegg.com
Solved Exp. 1 Beers' Law NAME PreLab Assignment Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the. Beer's Law Lab Chegg.
From www.chegg.com
Solved Lab Partner Instructor Experiment 10 Beer's Law Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. Beer's law is only valid at the. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. There are two contributions to this fundamental. . Beer's Law Lab Chegg.
From www.chegg.com
Solved Experiment 12 Beer's Law PreLab Report Sheet Name Beer's Law Lab Chegg calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Select the best statement about beer's law. beer’s law is a limiting law that is valid only for low concentrations of analyte. beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. Beer's law is. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law Name Date PRELAB QUESTIONS 1. What is the Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. There are two contributions to this fundamental. beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we. Beer's Law Lab Chegg.
From www.chegg.com
Solved Lab 5 2162 Beer's Law and Standard Curves Prepared by Beer's Law Lab Chegg since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Select the best statement about beer's law. beer's law states that the absorbance. Beer's Law Lab Chegg.
From www.chegg.com
Solved BEERS LAWTransmi COLORIMETRYBEER'S LAW LAB REPORT Beer's Law Lab Chegg Use the beer's law plot to determine the. beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. There are two contributions to this fundamental. Beer's law is only valid at the. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). beer’s law is. Beer's Law Lab Chegg.
From www.chegg.com
Solved Quantitative Chemical Analysis Beer's Law Lab Beer's Law Lab Chegg in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. There are two contributions to this fundamental.. Beer's Law Lab Chegg.
From www.chegg.com
Open the Beer's Law Lab simulation on your laptop or Beer's Law Lab Chegg There are two contributions to this fundamental. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its.. Beer's Law Lab Chegg.
From www.chegg.com
Spectrophotometry and Beer's Law spermanent Lab Beer's Law Lab Chegg calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Beer's law is only valid at the. beer’s law is a limiting law that is valid only for low concentrations of analyte. Use the beer's law plot to determine the. beer's law the goal of this experiment is to determine the concentration of nickel. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law Lab Leaming Objective 1) Develop a Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. beer’s law is a limiting law that is valid only for. Beer's Law Lab Chegg.
From www.chegg.com
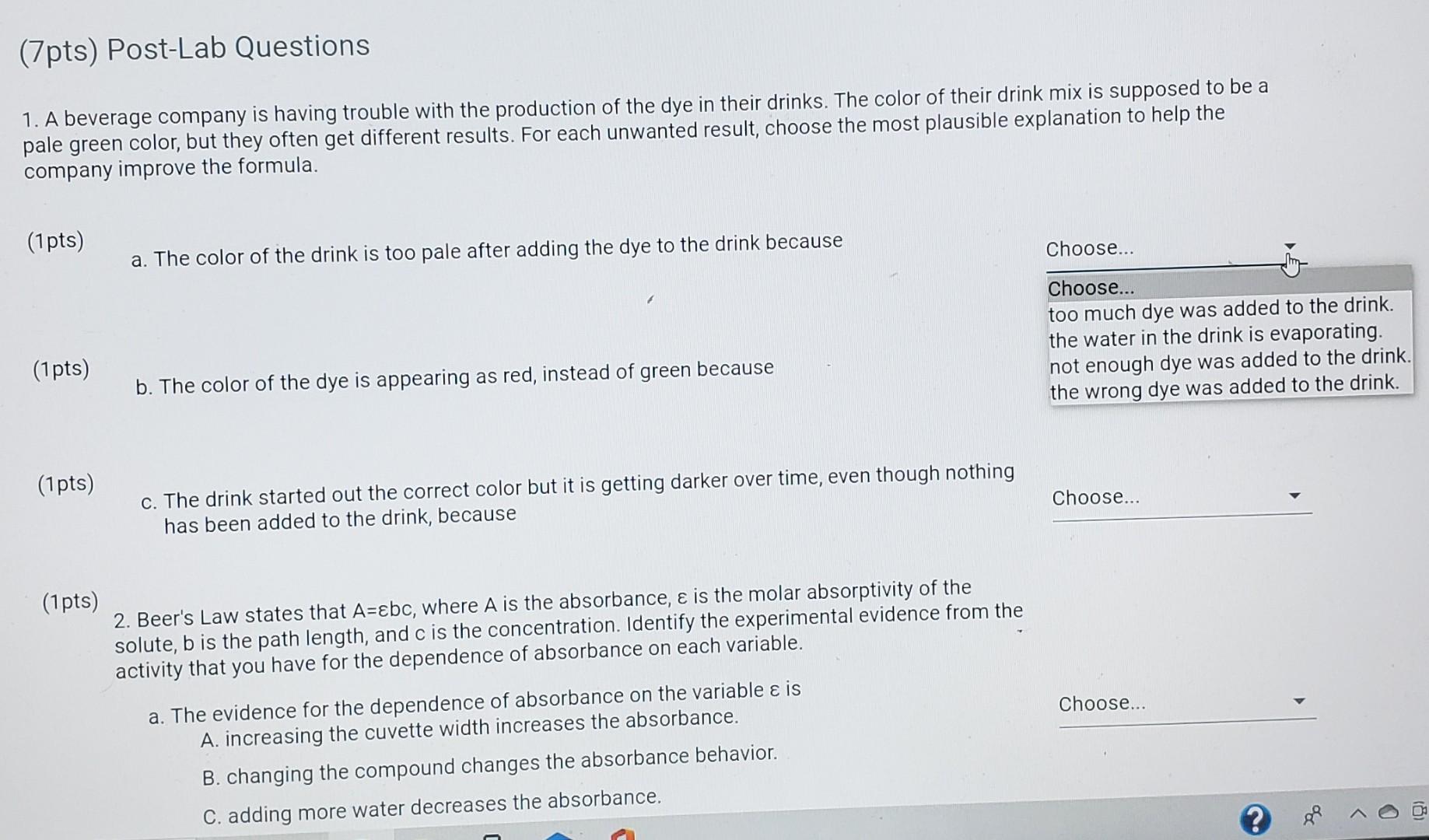
Solved 2. (7pts) In the Beer's law lab, a student got a set Beer's Law Lab Chegg calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the beer's law plot to determine the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law is a limiting law that is valid only for low concentrations of analyte. Beer's. Beer's Law Lab Chegg.
From www.chegg.com
Solved 2. Without correcting dilution in a beer's law lab Beer's Law Lab Chegg calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. There are two contributions to this fundamental. beer’s law is a limiting law that is valid only for low concentrations of analyte. Use the. Beer's Law Lab Chegg.
From www.chegg.com
Solved SPECTROPHOTOMETRY. APPLY BEER'S LAW TO DETERMINE Beer's Law Lab Chegg calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). There are two contributions to this fundamental. beer’s law is a limiting law that is valid only for low concentrations of analyte. Select the best statement about beer's law. Beer's law is only valid at the. since the concentration, path length and molar absorptivity. Beer's Law Lab Chegg.
From www.chegg.com
Solved Simulation Data and Report Submission Beer's Law Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. Select the best statement about beer's law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law is a limiting law that is valid only for. Beer's Law Lab Chegg.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Lab Chegg Select the best statement about beer's law. Beer's law is only valid at the. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). There are two contributions to this fundamental.. Beer's Law Lab Chegg.
From www.chegg.com
Solved Post Lab Questions Beer's Law (20 points) Answer the Beer's Law Lab Chegg calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Select the best statement about beer's law. Use the beer's law plot to determine the. There are two contributions to this fundamental. beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. since the concentration,. Beer's Law Lab Chegg.
From www.chegg.com
Open the PhET lab simulation for Beer's Law in a new Beer's Law Lab Chegg Use the beer's law plot to determine the. Select the best statement about beer's law. There are two contributions to this fundamental. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). in spectroscopy,. Beer's Law Lab Chegg.
From www.chegg.com
Solved Simulation Data and Report Submission Beer's Law Beer's Law Lab Chegg since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. beer’s law is a limiting law that is valid only for low concentrations of analyte. Use the beer's law plot to determine the. Select the best statement about beer's law. beer's law the goal of this experiment. Beer's Law Lab Chegg.
From www.chegg.com
Open the Beer's Law Lab simulation on your laptop or Beer's Law Lab Chegg There are two contributions to this fundamental. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity. Beer's Law Lab Chegg.
From www.chegg.com
Solved none Beers Law Lab PhET INTERACTIVE SIMULATIONS Beer's Law Lab Chegg There are two contributions to this fundamental. Beer's law is only valid at the. Select the best statement about beer's law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law).. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law Lab Chegg since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Use the beer's law plot to determine the. Select the best statement about beer's law. There are two contributions to this fundamental. beer's law the goal of this experiment is to determine the concentration of nickel ions in. Beer's Law Lab Chegg.
From www.chegg.com
Solved Where will you get the absorbance readings? Beer's Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. There are two contributions to this fundamental. Beer's law is only valid at the. Select the best statement about beer's law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the. Beer's Law Lab Chegg.
From www.chegg.com
Solved Prelab LO7 Beer's Law (10pts) Name Please review the Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the beer's law plot to determine the. Select the best statement about beer's law. There are two contributions to this fundamental. in spectroscopy, beer’s. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law Lab Leaming Objective 1) Develop a Beer's Law Lab Chegg beer’s law is a limiting law that is valid only for low concentrations of analyte. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law is only valid at the. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). There are. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law Part 2 Concentration from Color Data & Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. Beer's law is only valid at the. There are two contributions to this fundamental. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and. Beer's Law Lab Chegg.
From www.chegg.com
Solved Using the interactive simulation, Beer's Law Lab, Beer's Law Lab Chegg since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. There are two contributions to. Beer's Law Lab Chegg.
From www.chegg.com
Solved Lab 5 2162 Beer's Law and Standard Curves Prepared by Beer's Law Lab Chegg Use the beer's law plot to determine the. beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. Beer's law is only valid at the. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution. Beer's Law Lab Chegg.
From www.chegg.com
Solved Lab Report Beer's Law Part 1 Determination of the Beer's Law Lab Chegg There are two contributions to this fundamental. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution and the path. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its.. Beer's Law Lab Chegg.
From www.chegg.com
Experiment 12 Beer's Law Lab Report Sheet Name Beer's Law Lab Chegg Use the beer's law plot to determine the. Select the best statement about beer's law. There are two contributions to this fundamental. Beer's law is only valid at the. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. calculate the concentration of a. Beer's Law Lab Chegg.
From www.chegg.com
A 3 paged lab over the Beer's Law screen. Its a quick Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Select the best statement about beer's law. beer’s law is a limiting law that. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law Lab Chegg beer's law the goal of this experiment is to determine the concentration of nickel ions in an unknown solution. Use the beer's law plot to determine the. Beer's law is only valid at the. beer's law states that the absorbance (a) of a solution is directly proportional to the concentration (c) of the absorbing species in the solution. Beer's Law Lab Chegg.
From www.chegg.com
Solved Beer's Law lab Answer & Show work please Part A 1) Beer's Law Lab Chegg calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Use the beer's law plot to determine the. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional. Beer's Law Lab Chegg.