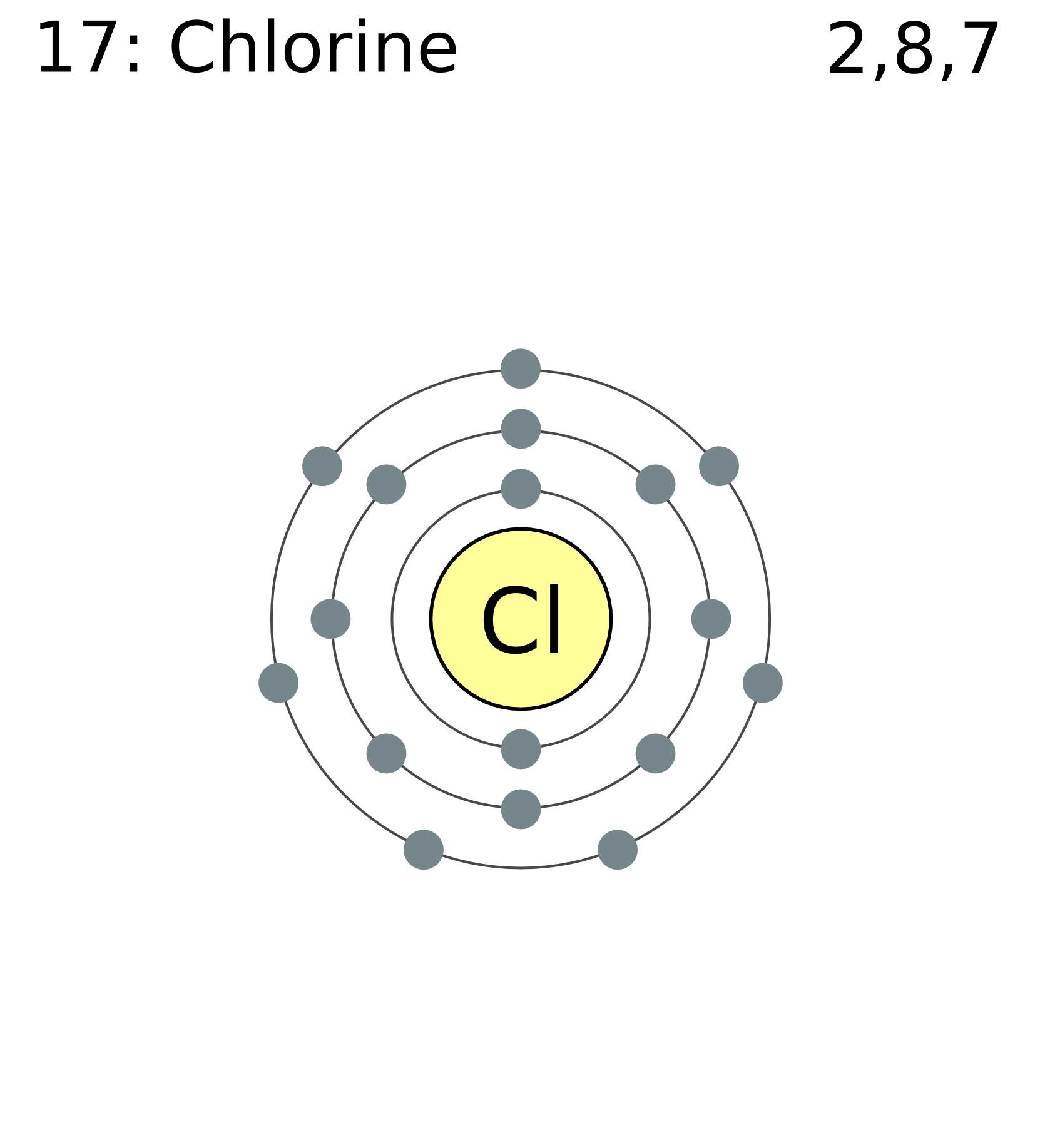
Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure . Each h atom (group 1) has 1 valence electron, and the. In the periodic table, chlorine is a group viia element with seven electrons in its last shell. The lewis electron dot diagram would look like the following: A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Determine the total number of valence electrons in the molecule or ion. In the lewis dot diagram of. The chlorine molecule contains two chlorine atoms. Therefore, the total number of valence. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. In a picture, the valence. Therefore, chlorine has 7 valence electrons. This leads us to conclude that br has 17 valence electrons, which makes it. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons.
from commons.wikimedia.org
Therefore it has seven valence electrons and needs to have seven dots drawn around its. The chlorine molecule contains two chlorine atoms. Each h atom (group 1) has 1 valence electron, and the. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. In a picture, the valence. In the periodic table, chlorine is a group viia element with seven electrons in its last shell. Determine the total number of valence electrons in the molecule or ion. The lewis electron dot diagram would look like the following: This leads us to conclude that br has 17 valence electrons, which makes it. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons.
FileElectron shell 017 chlorine.png Wikimedia Commons
Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore, the total number of valence. Therefore, the total number of valence. Determine the total number of valence electrons in the molecule or ion. Therefore, chlorine has 7 valence electrons. In the periodic table, chlorine is a group viia element with seven electrons in its last shell. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. The lewis electron dot diagram would look like the following: Each h atom (group 1) has 1 valence electron, and the. The chlorine molecule contains two chlorine atoms. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. This leads us to conclude that br has 17 valence electrons, which makes it. Therefore it has seven valence electrons and needs to have seven dots drawn around its. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. In the lewis dot diagram of. In a picture, the valence.
From valenceelectrons.com
How Many Valence Electrons Does Aluminum Chloride Have? Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In a picture, the valence. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. In the lewis dot diagram of. Determine the total number of valence electrons in the molecule or ion. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.chemtopper.com
How to Draw Lewis Dot Structure Online Chemistry Tutor Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In a picture, the valence. The chlorine molecule contains two chlorine atoms. Determine the total number of valence electrons in the molecule or ion. In the lewis dot diagram of. Each h atom (group 1) has 1 valence electron, and the. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. A lewis electron dot diagram. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.ck12.org
Valence Electrons CK12 Foundation Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore, chlorine has 7 valence electrons. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. Determine the total number of valence electrons in the molecule or ion. The lewis electron dot diagram would look like the following: In the lewis dot diagram of. Therefore it has seven valence electrons and. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.sliderbase.com
Valence Electrons Presentation Chemistry Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore, chlorine has 7 valence electrons. Each h atom (group 1) has 1 valence electron, and the. The chlorine molecule contains two chlorine atoms. In the lewis dot diagram of. In a picture, the valence. In the periodic table, chlorine is a group viia element with seven electrons in its last shell. Therefore it has seven valence electrons and needs. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore, chlorine has 7 valence electrons. Determine the total number of valence electrons in the molecule or ion. Each h atom (group 1) has 1 valence electron, and the. This leads us to conclude that br has 17 valence electrons, which makes it. The lewis electron dot diagram would look like the following: In the lewis dot diagram of. The. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From hanenhuusholli.blogspot.com
Lewis Dot Diagram For Chlorine Hanenhuusholli Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. In a picture, the valence. The lewis electron dot diagram would look like the following: Therefore, chlorine has 7 valence electrons. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.youtube.com
How many valence electrons are in a chlorine atom YouTube Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Each h atom (group 1) has 1 valence electron, and the. The lewis electron dot diagram would look like the following: A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Therefore it has seven valence electrons and needs to have seven dots drawn around its. This leads. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore it has seven valence electrons and needs to have seven dots drawn around its. The chlorine molecule contains two chlorine atoms. In a picture, the valence. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Therefore, chlorine has 7 valence electrons. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram,. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.youtube.com
How many valence electrons does chlorine have?How to find the valence electrons for chlorine Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. In a picture, the valence. The chlorine molecule contains two chlorine atoms. Determine the total number of valence electrons in the molecule or ion. Each h atom. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From modernizemodest1712.blogspot.com
40 how many electrons are depicted in the electron dot diagram Diagram For You Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure The chlorine molecule contains two chlorine atoms. This leads us to conclude that br has 17 valence electrons, which makes it. Determine the total number of valence electrons in the molecule or ion. Therefore, chlorine has 7 valence electrons. The lewis electron dot diagram would look like the following: In the periodic table, chlorine is a group viia element with. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Determine the total number of valence electrons in the molecule or ion. This leads us to conclude that br has 17 valence electrons, which makes it. Therefore, chlorine has 7 valence electrons. In the lewis dot diagram of. In the periodic table, chlorine is a group viia element with seven electrons in its last shell. In a picture, the valence.. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure The chlorine molecule contains two chlorine atoms. Determine the total number of valence electrons in the molecule or ion. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. Therefore, the total number of valence. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Therefore it. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From printablelibtesty.z13.web.core.windows.net
Lewis Dot Structure Organic Chemistry Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. The lewis electron dot diagram would look like the following: This leads us to conclude that br has 17. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure This leads us to conclude that br has 17 valence electrons, which makes it. The chlorine molecule contains two chlorine atoms. Determine the total number of valence electrons in the molecule or ion. Therefore it has seven valence electrons and needs to have seven dots drawn around its. A lewis electron dot diagram (or electron dot diagram, or a lewis. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure The chlorine molecule contains two chlorine atoms. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. This leads us to conclude that br has 17 valence electrons, which makes it. Each h atom (group 1) has 1 valence electron, and the. In a picture, the valence. Chlorine has seven valence electrons, which are the electrons. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From circuitdiagramapplied.z21.web.core.windows.net
Lewis Dot Diagram For First 20 Elements Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In the periodic table, chlorine is a group viia element with seven electrons in its last shell. In a picture, the valence. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Therefore, chlorine has 7 valence electrons. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure). Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In the lewis dot diagram of. Determine the total number of valence electrons in the molecule or ion. The chlorine molecule contains two chlorine atoms. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Therefore, the total number of valence. Each h atom (group 1) has 1 valence electron, and the. The lewis electron. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From brainly.com
Select the correct answer. How many valence electrons does the Lewis structure for a chlorine Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In the periodic table, chlorine is a group viia element with seven electrons in its last shell. This leads us to conclude that br has 17 valence electrons, which makes it. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. The chlorine molecule contains two chlorine atoms. In a picture, the valence. A lewis electron. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure This leads us to conclude that br has 17 valence electrons, which makes it. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. Therefore it has seven valence electrons and needs to have seven dots drawn around its. In the periodic table, chlorine is a group viia element with seven. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In the lewis dot diagram of. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. In a picture, the valence. Therefore, the total number of valence. Therefore, chlorine has 7 valence electrons. In the periodic table, chlorine is a group viia element with seven electrons in its last shell. This leads us to conclude that. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From schematicpadaczkai6.z19.web.core.windows.net
Lewis Dot Diagram For The First 20 Elements Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In the periodic table, chlorine is a group viia element with seven electrons in its last shell. In a picture, the valence. Therefore, the total number of valence. Each h atom (group 1) has 1 valence electron, and the. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.expii.com
Valence Electrons — Definition & Importance Expii Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In the periodic table, chlorine is a group viia element with seven electrons in its last shell. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. In a picture, the valence. Therefore, chlorine. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. In the lewis dot diagram of. Determine the total number of valence electrons in the molecule or ion. The lewis electron dot diagram would. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From sciencenotes.org
How to Draw a Lewis Structure Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. The chlorine molecule contains two chlorine atoms. Therefore, chlorine has 7 valence electrons. Determine the total number of valence electrons in the molecule or ion. Each h atom (group 1) has 1 valence electron, and the. Chlorine has. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure This leads us to conclude that br has 17 valence electrons, which makes it. In the lewis dot diagram of. Therefore it has seven valence electrons and needs to have seven dots drawn around its. In a picture, the valence. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Chlorine has seven valence electrons, which. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore it has seven valence electrons and needs to have seven dots drawn around its. In a picture, the valence. Each h atom (group 1) has 1 valence electron, and the. The chlorine molecule contains two chlorine atoms. Therefore, the total number of valence. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From slideplayer.com
Chemistry for the Biologist ppt download Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In a picture, the valence. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Therefore, the total number of valence. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. In the lewis dot diagram of. This leads us to conclude that br has 17 valence. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure The chlorine molecule contains two chlorine atoms. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. In the lewis dot diagram of. Therefore, the total number of valence. This leads us to conclude that br has 17 valence electrons, which makes it. Determine the total number of. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore, chlorine has 7 valence electrons. Determine the total number of valence electrons in the molecule or ion. In the lewis dot diagram of. In a picture, the valence. This leads us to conclude that br has 17 valence electrons, which makes it. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. The lewis electron. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Determine the total number of valence electrons in the molecule or ion. Each h atom (group 1) has 1 valence electron, and the. Chlorine has seven valence electrons, which are the electrons in the outermost energy level or shell of an atom. Therefore, the total number of valence. Therefore it has seven valence electrons and needs to have seven dots. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence electrons in chlorine Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Determine the total number of valence electrons in the molecule or ion. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. The chlorine molecule contains two chlorine atoms. This leads us to conclude that br has 17 valence electrons, which makes it. Each h atom (group 1). Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Each h atom (group 1) has 1 valence electron, and the. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Determine the total number of valence electrons in the molecule or ion. Therefore, chlorine has 7 valence electrons. The #3s^2 3p^5# electrons are the outermost electrons, so. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure In the lewis dot diagram of. In a picture, the valence. The chlorine molecule contains two chlorine atoms. The #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Determine the total number of valence electrons in the molecule or ion. A. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From www.doubtnut.com
In electron dot structure, The valence shell electrons are represented Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Therefore, chlorine has 7 valence electrons. Therefore, the total number of valence. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Each h atom (group 1) has 1 valence electron, and the. This leads us to conclude that br has 17 valence electrons, which makes it. Determine the total number of valence electrons in. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.
From brainly.in
Draw the electron dot structure of chlorine molecule Brainly.in Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure Each h atom (group 1) has 1 valence electron, and the. The lewis electron dot diagram would look like the following: In the periodic table, chlorine is a group viia element with seven electrons in its last shell. Therefore, chlorine has 7 valence electrons. The chlorine molecule contains two chlorine atoms. The #3s^2 3p^5# electrons are the outermost electrons, so. Chlorine Should Have How Many Valence Electrons Of How Many Dots Are On Lewis Dot Structure.