Why Boil Water When Pressure Is Low . Watch water boil at room temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Clear plastic syringe (10 ml works well) water. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. The water holds less heat so proper cooking requires more time. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; The change from a liquid phase to. The temperature at which water boils depends on pressure. Eventually, the water may cool to a temperature at which the vapor pressure is. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. As the ambient air pressure decreases, the temperature required to boil a liquid also decreases.
from pediaa.com
The temperature at which water boils depends on pressure. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Eventually, the water may cool to a temperature at which the vapor pressure is. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. The change from a liquid phase to. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. As the ambient air pressure decreases, the temperature required to boil a liquid also decreases. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. Watch water boil at room temperature.
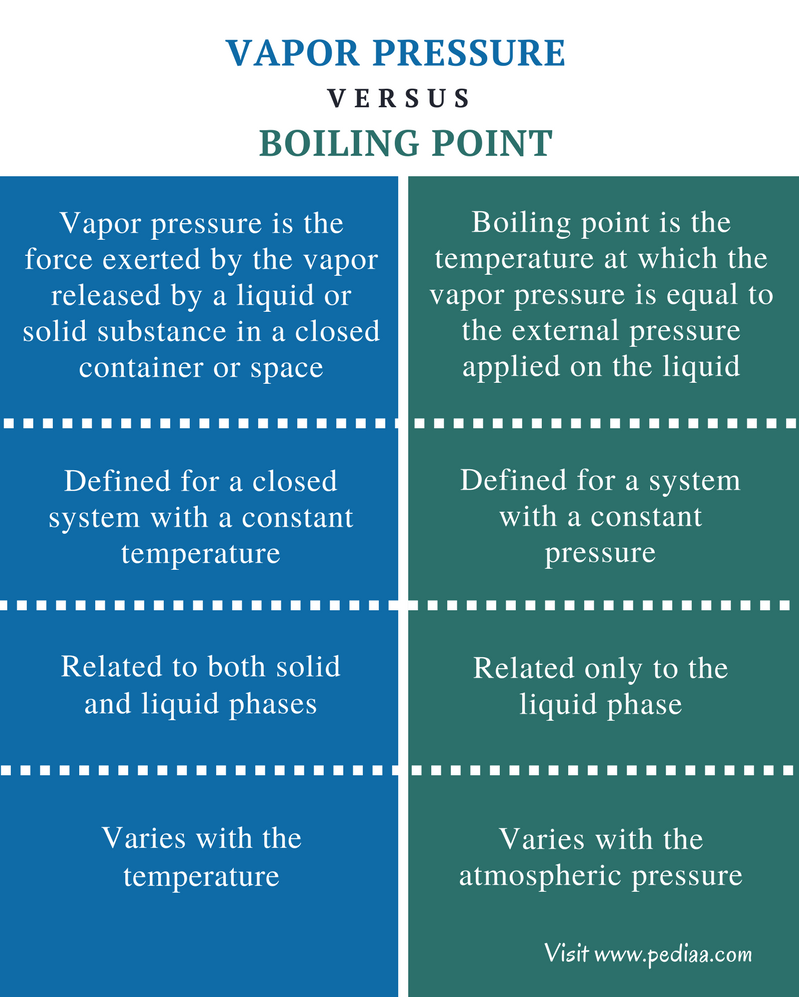
Difference Between Vapor Pressure and Boiling Point Definition
Why Boil Water When Pressure Is Low Watch water boil at room temperature. The water holds less heat so proper cooking requires more time. The temperature at which water boils depends on pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. Eventually, the water may cool to a temperature at which the vapor pressure is. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; Clear plastic syringe (10 ml works well) water. Watch water boil at room temperature. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. As the ambient air pressure decreases, the temperature required to boil a liquid also decreases. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol.
From waterpurificationguide.com
Why Is Water Pressure Low In My House? Water Purification Guide Why Boil Water When Pressure Is Low For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; Clear plastic syringe (10 ml works well) water. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Eventually, the water may cool to a temperature at which the vapor pressure is. Watch water. Why Boil Water When Pressure Is Low.
From www.youtube.com
Low Pressure Boiling YouTube Why Boil Water When Pressure Is Low Eventually, the water may cool to a temperature at which the vapor pressure is. The water holds less heat so proper cooking requires more time. Watch water boil at room temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, it takes longer to make some foods. Why Boil Water When Pressure Is Low.
From mavink.com
Water Boiling Point Pressure Chart Why Boil Water When Pressure Is Low The temperature at which water boils depends on pressure. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; The change from a liquid. Why Boil Water When Pressure Is Low.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Why Boil Water When Pressure Is Low For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; The temperature at which water boils depends on pressure. The change from a liquid phase to. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. Boiling is the process by which a liquid turns. Why Boil Water When Pressure Is Low.
From www.branchor.com
Why Is My Water Pressure Low? Top 5 Reasons and How to Fix Them The Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. The temperature at which water boils depends on pressure. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. Clear plastic syringe (10 ml. Why Boil Water When Pressure Is Low.
From www.youtube.com
How To Identify and Correct Low Water Pressure YouTube Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; Watch water boil at room temperature. As. Why Boil Water When Pressure Is Low.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Clear plastic syringe (10 ml works well) water. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, it takes longer to make some foods at high elevations because water. Why Boil Water When Pressure Is Low.
From www.kingsservices.com
Low Home Water Pressure 8 Causes and How to Fix It Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. The change from a liquid phase to. As the ambient air pressure decreases, the temperature required to boil a liquid also decreases. Boiling is the. Why Boil Water When Pressure Is Low.
From worksheetlistyu.z13.web.core.windows.net
Vapor Pressure And Boiling Worksheets Why Boil Water When Pressure Is Low The change from a liquid phase to. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. The temperature at which water boils depends on pressure. Clear plastic syringe (10 ml works well) water. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. For. Why Boil Water When Pressure Is Low.
From blog.mavyn.com
Why is My Water Pressure Low? Why Boil Water When Pressure Is Low As the ambient air pressure decreases, the temperature required to boil a liquid also decreases. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. The change from a liquid phase to. Boiling occurs when the vapor pressure of the hot liquid. Why Boil Water When Pressure Is Low.
From www.animalia-life.club
Boiling Point Of Water For Kids Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Eventually, the water may cool to a temperature at which the vapor pressure is. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; Boiling occurs when the vapor pressure of the hot liquid. Why Boil Water When Pressure Is Low.
From www.youtube.com
Boiling point and external pressure YouTube Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. The water holds less heat so proper cooking requires more time. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. As the water boils, heat is lost due to the heat. Why Boil Water When Pressure Is Low.
From www.wallshq.com
Possible Causes of Low Water Pressure Why Boil Water When Pressure Is Low The change from a liquid phase to. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. Clear plastic syringe (10 ml works well) water. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; The temperature at which water boils depends on. Why Boil Water When Pressure Is Low.
From www.dreamstyleremodeling.com
Causes of Low Water Pressure Dreamstyle Remodeling Why Boil Water When Pressure Is Low Eventually, the water may cool to a temperature at which the vapor pressure is. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the.. Why Boil Water When Pressure Is Low.
From www.stpaulpipeworks.com
Low Water Pressure? Here’s what’s wrong and how to fix it Why Boil Water When Pressure Is Low Watch water boil at room temperature. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; As the ambient air pressure decreases, the temperature required to boil a liquid also decreases. As the water boils,. Why Boil Water When Pressure Is Low.
From www.your1plumberfl.com
Low Water Pressure Causes That You Should Know Blog Your 1 Plumber FL Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. The change from a liquid phase to. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have. Why Boil Water When Pressure Is Low.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Why Boil Water When Pressure Is Low When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. Watch water boil at room temperature. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase. Why Boil Water When Pressure Is Low.
From sciencenotes.org
How to Boil Water at Room Temperature Why Boil Water When Pressure Is Low Eventually, the water may cool to a temperature at which the vapor pressure is. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. Watch water boil at room temperature. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. When the water. Why Boil Water When Pressure Is Low.
From www.youtube.com
Why Is My Water Pressure Low? 🔍 Essential Fixes & Tips! YouTube Why Boil Water When Pressure Is Low When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. The change from a liquid phase to. Watch water boil at room temperature.. Why Boil Water When Pressure Is Low.
From flohawks.com
Why Is My Water Pressure Low? FloHawks Plumbing + Septic Why Boil Water When Pressure Is Low When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. The change from a liquid phase to. Boiling is the process by which. Why Boil Water When Pressure Is Low.
From facts.net
20 Fascinating Facts About Boiling Point Why Boil Water When Pressure Is Low Watch water boil at room temperature. The temperature at which water boils depends on pressure. The change from a liquid phase to. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Eventually, the water may cool to a temperature at which the vapor pressure is. Boiling occurs when the vapor pressure. Why Boil Water When Pressure Is Low.
From www.thespruce.com
Common Causes of Low Water Pressure Why Boil Water When Pressure Is Low As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. Watch water boil at room temperature. Eventually, the water may cool to a temperature at which the vapor pressure is. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Boiling is the. Why Boil Water When Pressure Is Low.
From www.plumbingforce.co.uk
Common Causes of Low Water Pressure And How To Fix Them Plumbing Force Why Boil Water When Pressure Is Low The temperature at which water boils depends on pressure. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; Eventually, the water may cool to a temperature at which the vapor pressure is. As. Why Boil Water When Pressure Is Low.
From www.slideserve.com
PPT Lab5 PowerPoint Presentation, free download ID2480710 Why Boil Water When Pressure Is Low Clear plastic syringe (10 ml works well) water. The temperature at which water boils depends on pressure. Watch water boil at room temperature. Eventually, the water may cool to a temperature at which the vapor pressure is. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. As the water boils, heat. Why Boil Water When Pressure Is Low.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Why Boil Water When Pressure Is Low When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. As the water boils, heat is lost due to the heat of vaporization of water,. Why Boil Water When Pressure Is Low.
From mungfali.com
Water Boiling Point Pressure Chart Why Boil Water When Pressure Is Low For example, it takes longer to make some foods at high elevations because water boils at lower temperatures; When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. Watch water boil at room temperature. Boiling is the process by which a liquid. Why Boil Water When Pressure Is Low.
From www.pureplumbinglv.com
Why Is My Water Pressure Low? How Do I Fix It? Pure Plumbing Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. When the water pressure drops below acceptable levels, the thousands of miles of very old underground pipe have cracks and holes that allow groundwater to seep into the. Watch water boil at room temperature. Eventually, the water may cool to a temperature. Why Boil Water When Pressure Is Low.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Eventually, the water may cool to a temperature at which the vapor pressure is. The temperature at which water boils depends on pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling. Why Boil Water When Pressure Is Low.
From www.witzamfm.com
Low Water Pressure Triggers Boil Water Advisory in Loogootee Why Boil Water When Pressure Is Low The water holds less heat so proper cooking requires more time. Watch water boil at room temperature. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. When the water pressure drops. Why Boil Water When Pressure Is Low.
From www.youtube.com
Vapor Pressure and Boiling YouTube Why Boil Water When Pressure Is Low The temperature at which water boils depends on pressure. The change from a liquid phase to. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Clear plastic syringe (10 ml works well) water. Watch water boil at room temperature. As the water boils, heat is lost due to the heat of. Why Boil Water When Pressure Is Low.
From www.plumbingbyjake.com
Why is My Water Pressure So Low? Plumbing By Jake Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. Watch water boil at room temperature. Clear plastic syringe (10 ml works well) water. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. The water holds less heat so proper cooking requires. Why Boil Water When Pressure Is Low.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs Why Boil Water When Pressure Is Low The temperature at which water boils depends on pressure. The change from a liquid phase to. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Clear plastic syringe (10 ml. Why Boil Water When Pressure Is Low.
From topforeignstocks.com
The Boiling Point of Water at Different Elevations Infographics Why Boil Water When Pressure Is Low An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kj/mol. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Watch water boil. Why Boil Water When Pressure Is Low.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Why Boil Water When Pressure Is Low Boiling occurs when the vapor pressure of the hot liquid equals the atmospheric pressure of its surroundings. The temperature at which water boils depends on pressure. Clear plastic syringe (10 ml works well) water. Eventually, the water may cool to a temperature at which the vapor pressure is. An educational demonstration on how boiling water behaves under reduced pressure, including. Why Boil Water When Pressure Is Low.
From mavink.com
Water Boiling Pressure Chart Why Boil Water When Pressure Is Low The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. An educational demonstration on how boiling water behaves under reduced pressure, including a microscopic view of bubble formation. For example, it takes longer to make some foods at high elevations because water boils. Why Boil Water When Pressure Is Low.