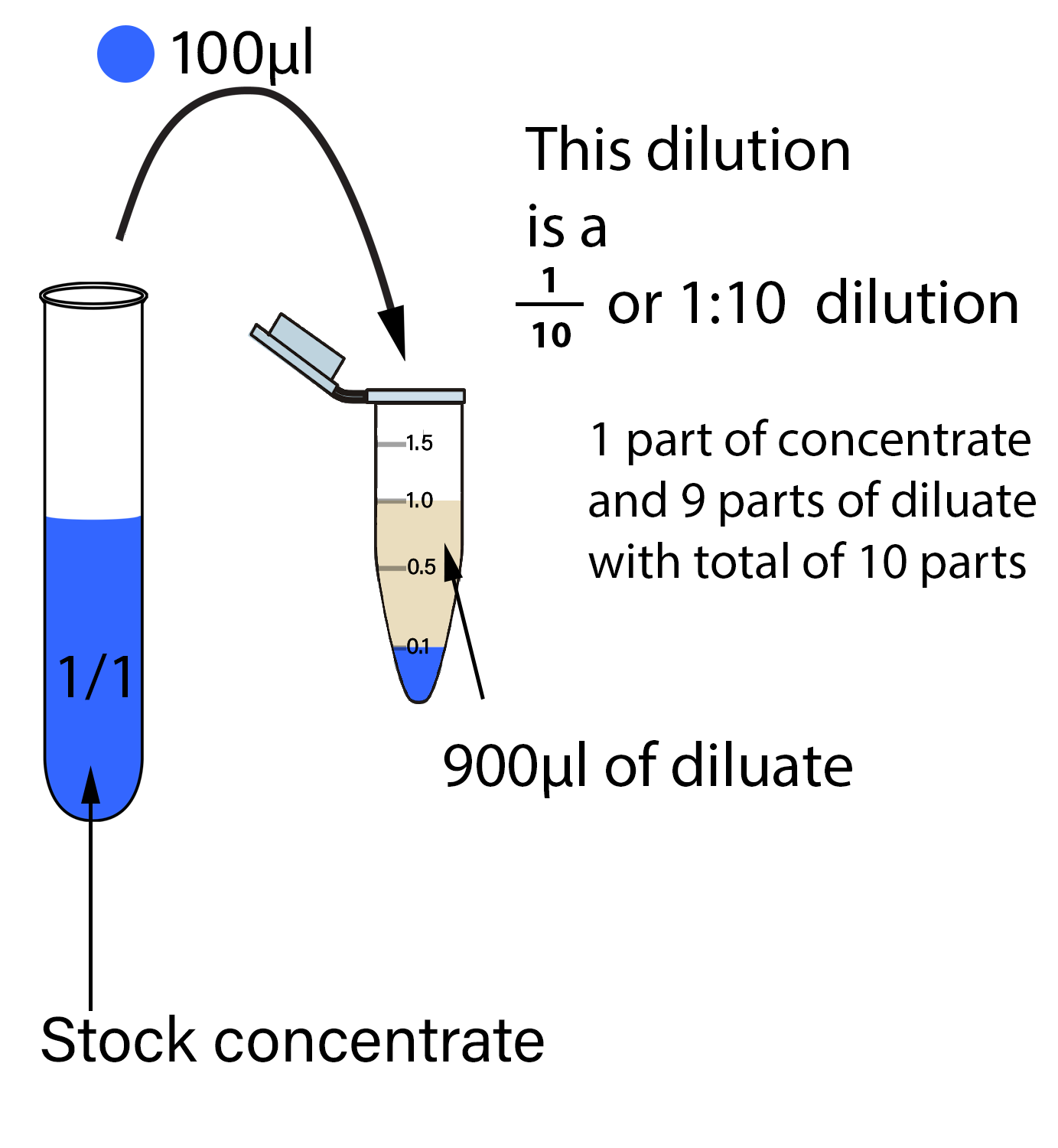
Dilution Well Meaning . There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,.
from www.medicine.mcgill.ca
There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Explanations and examples of common methods. We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory.
Serial Dilutions
Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. In both dilution and concentration,. Explanations and examples of common methods. Dilution is a process used to lower the concentration of the original solution by adding more solvent. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved in a given amount of.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Well Meaning Explanations and examples of common methods. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used. Dilution Well Meaning.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Well Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which increases the. Understand how stock solutions are used in the laboratory. We are often concerned. Dilution Well Meaning.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilution Well Meaning Dilution is a process used to lower the concentration of the original solution by adding more solvent. In both dilution and concentration,. Concentration is the removal of solvent, which increases the. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Well Meaning.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Well Meaning Dilution is a process used to lower the concentration of the original solution by adding more solvent. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the. There are many ways of expressing concentration. Dilution Well Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Well Meaning There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is. Dilution Well Meaning.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Well Meaning Dilution is a process used to lower the concentration of the original solution by adding more solvent. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. Understand how stock solutions are used in the laboratory. Dilution is the. Dilution Well Meaning.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Well Meaning There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Understand how stock. Dilution Well Meaning.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock. Dilution Well Meaning.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Well Meaning Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is a process used to lower the. Dilution Well Meaning.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. In both dilution and concentration,. Concentration is the removal of solvent, which increases the. Explanations and examples of common methods. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the. Dilution Well Meaning.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilution Well Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of. Dilution is a process used to lower the concentration of the original solution by adding more solvent. There are many. Dilution Well Meaning.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Well Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved in a given amount of. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the. Dilution Well Meaning.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Well Meaning Understand how stock solutions are used in the laboratory. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. In both dilution and concentration,. We are often concerned with how much solute is dissolved in a given amount of. Dilution is a process used to lower the concentration of the original solution by adding more. Dilution Well Meaning.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram Dilution Well Meaning In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the. There are many ways of expressing concentration and dilution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Understand how stock solutions. Dilution Well Meaning.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilution Well Meaning Understand how stock solutions are used in the laboratory. In both dilution and concentration,. We are often concerned with how much solute is dissolved in a given amount of. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the. Dilution is a process used to lower. Dilution Well Meaning.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Well Meaning Dilution is a process used to lower the concentration of the original solution by adding more solvent. In both dilution and concentration,. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute. Dilution Well Meaning.
From www.slideshare.net
Chemistry Chp 16 Solutions PowerPoint (shortened) Dilution Well Meaning In both dilution and concentration,. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the. Dilution Well Meaning.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilution Well Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the. Understand how stock. Dilution Well Meaning.
From microbeonline.com
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Dilution Well Meaning Explanations and examples of common methods. Concentration is the removal of solvent, which increases the. There are many ways of expressing concentration and dilution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in. Dilution Well Meaning.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Well Meaning Concentration is the removal of solvent, which increases the. There are many ways of expressing concentration and dilution. Explanations and examples of common methods. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often. Dilution Well Meaning.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Well Meaning Dilution is a process used to lower the concentration of the original solution by adding more solvent. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute. Dilution Well Meaning.
From extension.uga.edu
Understanding Laboratory Wastewater Tests I. Organics (BOD, COD, TOC Dilution Well Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration. Dilution Well Meaning.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. In both dilution and concentration,. Dilution is the addition of solvent,. Dilution Well Meaning.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilution Well Meaning Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. We are often concerned with how much solute is dissolved in a given amount of. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the.. Dilution Well Meaning.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilution Well Meaning Dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which increases the. In both dilution and concentration,. Explanations and examples of common methods. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases. Dilution Well Meaning.
From bryantkruwnavarro.blogspot.com
When Diluting a Solution Which of the Following Changes BryantkruwNavarro Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. In both dilution and concentration,. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the. Dilution is the addition of. Dilution Well Meaning.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. In both dilution and concentration,. Concentration is the removal of solvent, which increases the. Understand how stock solutions are used. Dilution Well Meaning.
From www.slashdotblog.com
What Is Dilution? Dilution Well Meaning Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. Dilution is the addition of solvent,. Dilution Well Meaning.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Understand how stock solutions are used in. Dilution Well Meaning.
From www.actioncleanup.com
Dilution Action’s Guide to Mixing the Right Solutions Dilution Well Meaning Dilution is a process used to lower the concentration of the original solution by adding more solvent. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. Explanations and examples of common methods. Concentration. Dilution Well Meaning.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Well Meaning Explanations and examples of common methods. Understand how stock solutions are used in the laboratory. In both dilution and concentration,. Dilution is a process used to lower the concentration of the original solution by adding more solvent. There are many ways of expressing concentration and dilution. We are often concerned with how much solute is dissolved in a given amount. Dilution Well Meaning.
From animalia-life.club
Solutions Examples Dilution Well Meaning In both dilution and concentration,. There are many ways of expressing concentration and dilution. Concentration is the removal of solvent, which increases the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition. Dilution Well Meaning.
From kerrlab.org
Kerr Wiki Public/Dilution Series Dilution Well Meaning Explanations and examples of common methods. Concentration is the removal of solvent, which increases the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Understand how stock solutions are used in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned. Dilution Well Meaning.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID6016027 Dilution Well Meaning There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. Concentration is the removal of solvent, which increases the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. We are often. Dilution Well Meaning.
From www.researchgate.net
MPN Decimal Dilution for the Well Water Samples Download Scientific Dilution Well Meaning We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used in the laboratory. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a process used to lower the concentration of the original solution by adding. Dilution Well Meaning.