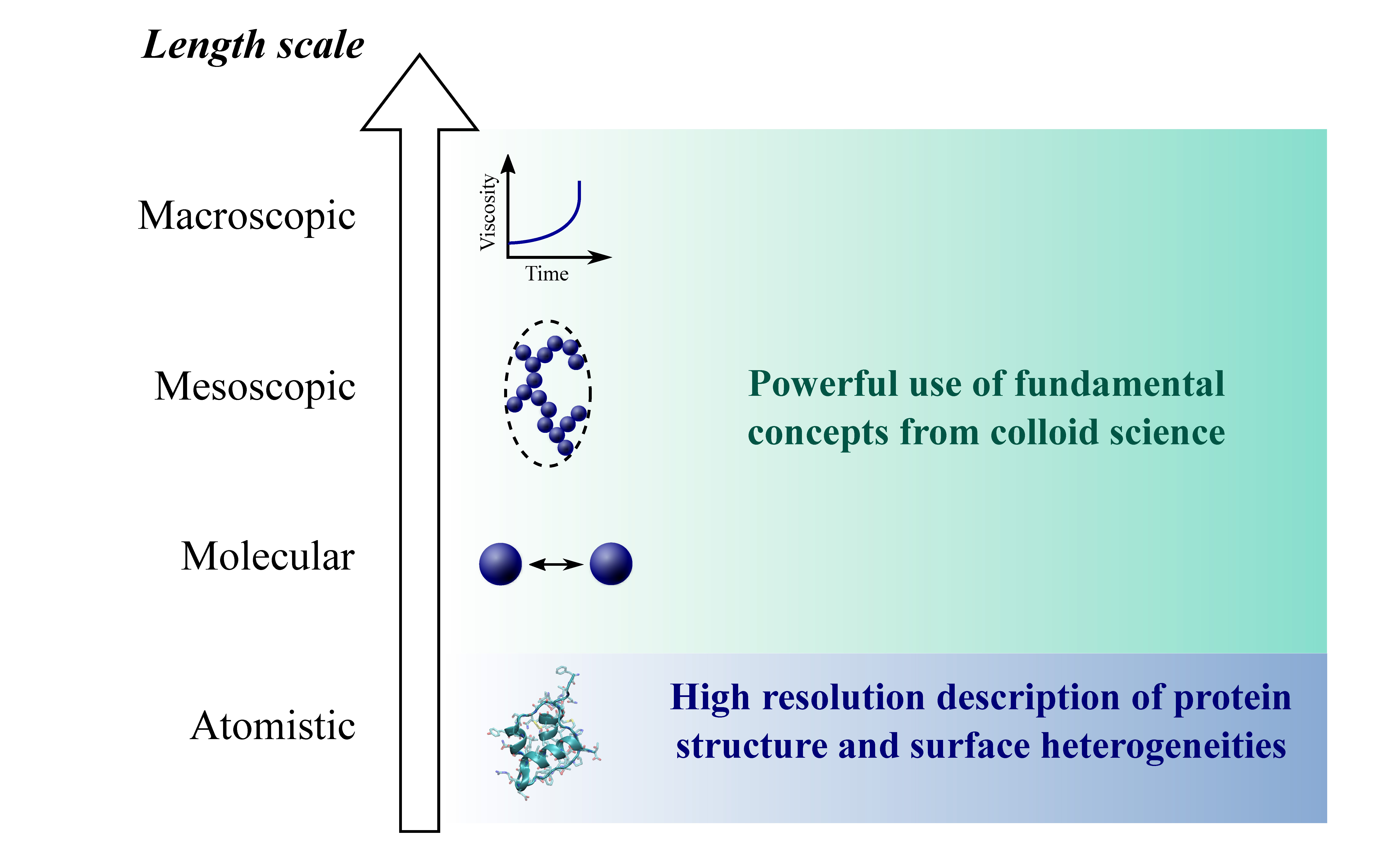
Unwanted Protein Aggregation . Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Globular soluble proteins, fibrous proteins, transmembrane. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein architectures can be categorized in four main classes: Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Protein misfolding and aggregation are linked to numerous human diseases and the. Protein aggregation is a common characteristic of many neurodegenerative diseases.
from morbidelli-group.ethz.ch
Protein aggregation is a common characteristic of many neurodegenerative diseases. Protein architectures can be categorized in four main classes: Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Globular soluble proteins, fibrous proteins, transmembrane. Protein misfolding and aggregation are linked to numerous human diseases and the. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein.
Aggregation of therapeutic proteins from dilute to concentrated
Unwanted Protein Aggregation Protein aggregation is a common characteristic of many neurodegenerative diseases. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Globular soluble proteins, fibrous proteins, transmembrane. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Protein architectures can be categorized in four main classes: Protein aggregation is a common characteristic of many neurodegenerative diseases. Protein misfolding and aggregation are linked to numerous human diseases and the.
From www.semanticscholar.org
[PDF] Therapeutic Approaches for Inhibition of Protein Aggregation in Unwanted Protein Aggregation Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid. Unwanted Protein Aggregation.
From www.researchgate.net
Protein aggregation and purity before and after protein stability Unwanted Protein Aggregation Globular soluble proteins, fibrous proteins, transmembrane. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Protein misfolding and aggregation are linked to numerous human diseases and the. Although microglia perform. Unwanted Protein Aggregation.
From www.researchgate.net
Protein Aggregation Accumulated in Cells. Protein aggregates were Unwanted Protein Aggregation Protein misfolding and aggregation are linked to numerous human diseases and the. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein aggregation. Unwanted Protein Aggregation.
From www.researchgate.net
Showing different factors affecting process of protein aggregation both Unwanted Protein Aggregation Protein misfolding and aggregation are linked to numerous human diseases and the. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Protein aggregation is a common characteristic of many neurodegenerative diseases. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can. Unwanted Protein Aggregation.
From www.researchgate.net
Overview of the modes of protein aggregation, problems associated with Unwanted Protein Aggregation Protein aggregation is a common characteristic of many neurodegenerative diseases. Globular soluble proteins, fibrous proteins, transmembrane. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein.. Unwanted Protein Aggregation.
From morbidelli-group.ethz.ch
Aggregation of therapeutic proteins from dilute to concentrated Unwanted Protein Aggregation Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: One. Unwanted Protein Aggregation.
From www.researchgate.net
Overview of nonnative protein aggregation pathways. APRs, aggregation Unwanted Protein Aggregation Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Globular soluble proteins, fibrous proteins, transmembrane. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein aggregation is a common characteristic of many neurodegenerative diseases. Protein architectures can. Unwanted Protein Aggregation.
From biofisica.info
Protein aggregation Toxicity and function, two sides of the same coin Unwanted Protein Aggregation However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Protein aggregation is a common characteristic. Unwanted Protein Aggregation.
From www.degruyter.com
Sizebased Degradation of Therapeutic Proteins Mechanisms, Modelling Unwanted Protein Aggregation Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which. Unwanted Protein Aggregation.
From www.mdpi.com
IJMS Free FullText Mechanism of Suppression of Protein Aggregation Unwanted Protein Aggregation Protein misfolding and aggregation are linked to numerous human diseases and the. Protein architectures can be categorized in four main classes: Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Globular soluble proteins, fibrous proteins, transmembrane.. Unwanted Protein Aggregation.
From molecularneurodegeneration.biomedcentral.com
Regulated protein aggregation stress granules and neurodegeneration Unwanted Protein Aggregation Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated. Unwanted Protein Aggregation.
From www.mdpi.com
Diseases Free FullText Protein Misfolding and Aggregation in Unwanted Protein Aggregation Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Protein misfolding and aggregation are linked to numerous human diseases and the. However, if chaperone networks fail to refold a damaged protein,. Unwanted Protein Aggregation.
From www.mdpi.com
IJMS Free FullText Mechanism of Suppression of Protein Aggregation Unwanted Protein Aggregation Protein aggregation is a common characteristic of many neurodegenerative diseases. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Protein aggregates are clusters of misfolded. Unwanted Protein Aggregation.
From www.science.org
Does protein aggregation drive postmitotic tissue degeneration Unwanted Protein Aggregation Protein architectures can be categorized in four main classes: Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Protein misfolding and aggregation are linked to numerous human diseases and the. One direction is. Unwanted Protein Aggregation.
From encyclopedia.pub
Peptide/Protein SelfAssembly and Aggregation Encyclopedia MDPI Unwanted Protein Aggregation One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Globular soluble proteins, fibrous proteins, transmembrane. Protein misfolding and aggregation are linked to numerous human diseases and the. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction. Unwanted Protein Aggregation.
From www.researchgate.net
A simplified model that describes the conformational transitions of Unwanted Protein Aggregation Protein aggregation is a common characteristic of many neurodegenerative diseases. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Protein misfolding and aggregation are linked to numerous human diseases and the. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Living systems have. Unwanted Protein Aggregation.
From www.researchgate.net
Mutant PFN1 G118V aggregation and abnormal protein ubiquitination Unwanted Protein Aggregation Protein aggregation is a common characteristic of many neurodegenerative diseases. Protein misfolding and aggregation are linked to numerous human diseases and the. Globular soluble proteins, fibrous proteins, transmembrane. Protein architectures can be categorized in four main classes: However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: One direction. Unwanted Protein Aggregation.
From www.science.org
Molecular Chaperones in the Cytosol from Nascent Chain to Folded Unwanted Protein Aggregation One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein misfolding and aggregation are linked to numerous human diseases and the. Protein architectures can be categorized in four main classes: However, if chaperone networks fail to refold a damaged protein, there are two. Unwanted Protein Aggregation.
From www.researchgate.net
Overview of nonnative protein aggregation pathways. APRs, aggregation Unwanted Protein Aggregation Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Protein aggregation is a common characteristic of many neurodegenerative diseases. Protein architectures can be categorized in four main classes: Globular soluble proteins, fibrous proteins, transmembrane. Protein misfolding and aggregation are linked to numerous human diseases and the. One direction is protein degradation, and the other. Unwanted Protein Aggregation.
From www.researchgate.net
Validation of the protein aggregation assay. (A) Schematic diagram of Unwanted Protein Aggregation One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting. Unwanted Protein Aggregation.
From pubs.rsc.org
Copper binding and protein aggregation a journey from the brain to the Unwanted Protein Aggregation Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation. Unwanted Protein Aggregation.
From www.cell.com
How do protein aggregates escape quality control in neurodegeneration Unwanted Protein Aggregation However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Protein architectures can be categorized in four main classes: Protein misfolding and aggregation are linked to numerous human diseases and the. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into. Unwanted Protein Aggregation.
From www.researchgate.net
7 Overview of protein aggregation pathways. Step 1 partial or complete Unwanted Protein Aggregation One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein architectures can be categorized in four main classes: However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Although microglia perform a. Unwanted Protein Aggregation.
From www.slideserve.com
PPT ProteinMisfolding Diseases PHY6940 April 8 th 2009 PowerPoint Unwanted Protein Aggregation Protein misfolding and aggregation are linked to numerous human diseases and the. Protein aggregation is a common characteristic of many neurodegenerative diseases. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and. Unwanted Protein Aggregation.
From www.biorender.com
Protein Aggregation Pathways BioRender Science Templates Unwanted Protein Aggregation Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Globular soluble proteins, fibrous proteins, transmembrane. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: One direction is protein degradation, and the other is the aggregation pathway, in which misfolded. Unwanted Protein Aggregation.
From phys.org
How protein aggregation occurs in cells Unwanted Protein Aggregation Protein architectures can be categorized in four main classes: Protein misfolding and aggregation are linked to numerous human diseases and the. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Globular soluble proteins, fibrous proteins, transmembrane. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein. Unwanted Protein Aggregation.
From www.researchgate.net
Prevention and reversion of protein aggregation by chaperones and Unwanted Protein Aggregation Protein architectures can be categorized in four main classes: However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at. Unwanted Protein Aggregation.
From documentride5.pythonanywhere.com
How To Prevent Protein Aggregation Documentride5 Unwanted Protein Aggregation Protein architectures can be categorized in four main classes: Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Protein aggregation is a common characteristic of many neurodegenerative diseases. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils.. Unwanted Protein Aggregation.
From pubs.rsc.org
Review of the current state of protein aggregation inhibition from a Unwanted Protein Aggregation Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Protein architectures can be categorized in four main classes: Protein misfolding and aggregation are linked to numerous human diseases and the. Protein. Unwanted Protein Aggregation.
From documentride5.pythonanywhere.com
How To Prevent Protein Aggregation Documentride5 Unwanted Protein Aggregation Protein architectures can be categorized in four main classes: One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Protein aggregation is a common characteristic of many neurodegenerative diseases. Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at. Unwanted Protein Aggregation.
From www.mdpi.com
IJMS Free FullText Cellular Protein Aggregates Formation Unwanted Protein Aggregation Protein misfolding and aggregation are linked to numerous human diseases and the. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. Globular soluble proteins, fibrous proteins, transmembrane. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. Protein. Unwanted Protein Aggregation.
From www.researchgate.net
Mechanisms and consequences of protein misfolding and aggregation. In Unwanted Protein Aggregation Protein aggregation is a common characteristic of many neurodegenerative diseases. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. However, if chaperone networks fail to refold a damaged protein, there are two paths that such a protein molecule can take: One direction is protein degradation, and the other is. Unwanted Protein Aggregation.
From www.researchgate.net
Aggregation process (time) of misfolded proteins. (A) Schematic Unwanted Protein Aggregation Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Globular soluble proteins, fibrous proteins, transmembrane. Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Although microglia perform a predominant role in the removal of extracellular aggregated proteins, mounting evidence. One. Unwanted Protein Aggregation.
From www.researchgate.net
Schematic illustration of protein aggregation in amyloid disorders Unwanted Protein Aggregation Protein aggregates are clusters of misfolded proteins that accumulate in cells, often leading to cellular dysfunction and are implicated in. Globular soluble proteins, fibrous proteins, transmembrane. Protein misfolding and aggregation are linked to numerous human diseases and the. Protein aggregation is a common characteristic of many neurodegenerative diseases. However, if chaperone networks fail to refold a damaged protein, there are. Unwanted Protein Aggregation.
From www.researchgate.net
Schematic illustration of the different modes of protein aggregation Unwanted Protein Aggregation Living systems have adopted various strategies to avoid unwanted protein aggregation and amyloid formation at high internal protein. Protein aggregation is a common characteristic of many neurodegenerative diseases. One direction is protein degradation, and the other is the aggregation pathway, in which misfolded proteins assemble into dimers and oligomers, which then form amyloid fibrils. However, if chaperone networks fail to. Unwanted Protein Aggregation.