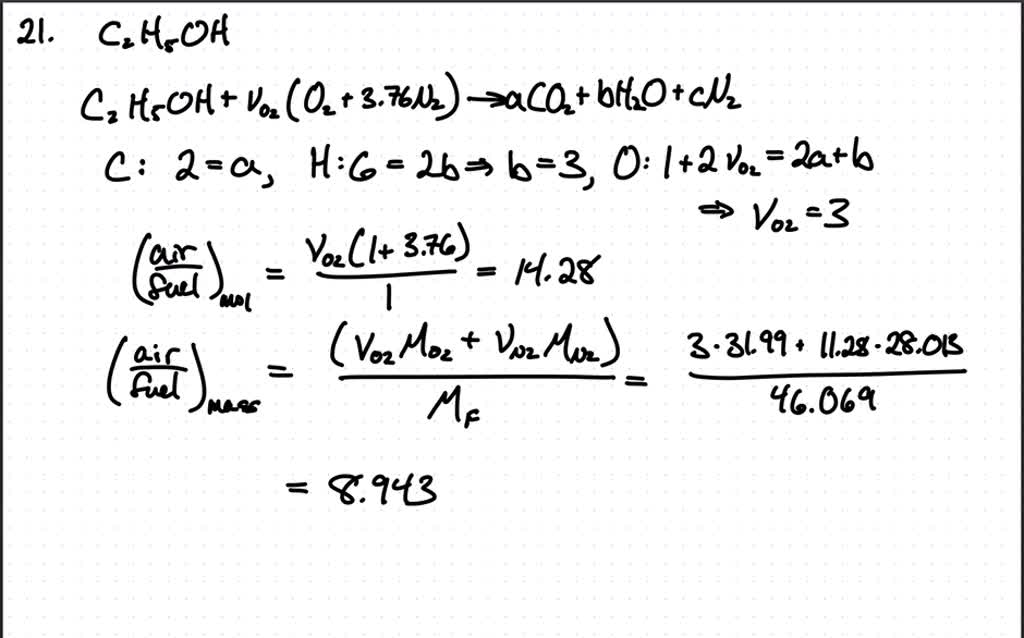
Stoichiometric Air Fuel Ratio For Butane . The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. What is stoichiometric air fuel ratio? The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. The above formula is for a single. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced.
from www.numerade.com
For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The above formula is for a single. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. What is stoichiometric air fuel ratio? The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air.
SOLVEDCalculate and compare the stoichiometric airfuel mass ratios
Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The above formula is for a single. What is stoichiometric air fuel ratio? For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air.
From www.scribd.com
9Stoichiometric air fuel ratio calculation for fuels12102023 PDF Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. The above formula is for a single. For example, if we have a mixture of methane and air which has the air. Stoichiometric Air Fuel Ratio For Butane.
From zander-jolpblogobrien.blogspot.com
Air Fuel Ratio in Gas Turbine Formula Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. What is stoichiometric air fuel ratio? Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Air Fuel ratio Stoichiometric air fuel ratio IC ENGINE YouTube Stoichiometric Air Fuel Ratio For Butane What is stoichiometric air fuel ratio? The above formula is for a single. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. For example, if we have a mixture of methane and air. Stoichiometric Air Fuel Ratio For Butane.
From inspectapedia.com
Definition and Importance of Stoichiometric Combustion Perfect Fuel Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. What is stoichiometric air fuel ratio? For example, if we have a mixture of methane and air which has the. Stoichiometric Air Fuel Ratio For Butane.
From www.coursehero.com
[Solved] Determine the stoichiometric airtofuel ratio for complete Stoichiometric Air Fuel Ratio For Butane The above formula is for a single. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. Air fuel ratio is defined as the ratio of air and fuel of. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Stoichiometric Air Fuel Ratio YouTube Stoichiometric Air Fuel Ratio For Butane The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. The above formula is for a single. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. What. Stoichiometric Air Fuel Ratio For Butane.
From www.scribd.com
Stoichiometric Air To Fuel Ratios of Common Fuels Sheet1 3 PDF Stoichiometric Air Fuel Ratio For Butane Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. The above formula is for a single. What is stoichiometric air fuel ratio? For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. When all the fuel is combined with. Stoichiometric Air Fuel Ratio For Butane.
From www.slideserve.com
PPT CHAPTER 12 GASOLINE, ALTERNATIVE FUELS, AND DIESEL FUELS Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The above formula is for a single. The stoichiometric air fuel ratio is the ratio that. Stoichiometric Air Fuel Ratio For Butane.
From www.numerade.com
SOLVED The stoichiometric combustion of butane (C4H10) in atmospheric Stoichiometric Air Fuel Ratio For Butane The above formula is for a single. What is stoichiometric air fuel ratio? For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. The stoichiometric air fuel ratio is the ratio that. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Air Fuel Ratio Explained Stoichiometric Ratio IC Engine Basics Stoichiometric Air Fuel Ratio For Butane Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it. Stoichiometric Air Fuel Ratio For Butane.
From x-engineer.org
Air fuel ratio Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared. Stoichiometric Air Fuel Ratio For Butane.
From www.coursehero.com
[Solved] Determine the stoichiometric airtofuel ratio for complete Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. What is stoichiometric air fuel ratio? Air fuel ratio is defined as the ratio of air. Stoichiometric Air Fuel Ratio For Butane.
From inspectapedia.com
Definition and Importance of Stoichiometric Combustion Perfect Fuel Stoichiometric Air Fuel Ratio For Butane For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The above formula is for a single. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. When all the fuel is combined with all the free oxygen, typically within. Stoichiometric Air Fuel Ratio For Butane.
From www.researchgate.net
Compartment air to fuel occurring and compared to stoichiometric Stoichiometric Air Fuel Ratio For Butane Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The above. Stoichiometric Air Fuel Ratio For Butane.
From www.coursehero.com
[Solved] Calculate the stoichiometric fuelair ratio on a volume basis Stoichiometric Air Fuel Ratio For Butane Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The amount. Stoichiometric Air Fuel Ratio For Butane.
From www.researchgate.net
Airfuel ratio at stoichiometric combustion Download Scientific Diagram Stoichiometric Air Fuel Ratio For Butane The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. The above formula is for a single. For example, if we have a mixture of methane and air which has. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Stoichiometric Air Fuel A F Ratio Combustion of Reactive Mixtures Stoichiometric Air Fuel Ratio For Butane The above formula is for a single. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. What is stoichiometric air fuel ratio? The stoichiometric air fuel ratio is the ratio that. Stoichiometric Air Fuel Ratio For Butane.
From www.slideserve.com
PPT ME 475/675 Introduction to Combustion PowerPoint Presentation Stoichiometric Air Fuel Ratio For Butane The above formula is for a single. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 31, pt 5 of 5 Air / Fuel Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air.. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Stoichiometric AirFuel Ratio Calculation YouTube Stoichiometric Air Fuel Ratio For Butane The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The above formula is for a single. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. What is stoichiometric air fuel ratio? Air fuel ratio is defined as the. Stoichiometric Air Fuel Ratio For Butane.
From shabnumbasant.blogspot.com
27+ air fuel ratio calculation ShabnumBasant Stoichiometric Air Fuel Ratio For Butane The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The above formula is for a single. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has. Stoichiometric Air Fuel Ratio For Butane.
From www.numerade.com
SOLVEDCalculate and compare the stoichiometric airfuel mass ratios Stoichiometric Air Fuel Ratio For Butane Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. What is stoichiometric air fuel ratio? The above formula is for a single. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. When all the fuel is combined with all the free oxygen,. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Numerical on Stoichiometric Air Fuel A F Ratio Combustion of Reactive Stoichiometric Air Fuel Ratio For Butane For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. What is stoichiometric air fuel ratio? Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. The above formula is for a single. The amount of air required for combusting. Stoichiometric Air Fuel Ratio For Butane.
From www.slideserve.com
PPT Lecture Objectives PowerPoint Presentation, free download ID Stoichiometric Air Fuel Ratio For Butane For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. The above formula is for a single. When all the fuel is combined with all the free oxygen, typically within a vehicle’s. Stoichiometric Air Fuel Ratio For Butane.
From www.numerade.com
For fuel oil with a weight composition of C86; H14, calculate the Stoichiometric Air Fuel Ratio For Butane For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. When all the fuel. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Air Fuel ratio Stoichiometric air fuel ratio IC ENGINE YouTube Stoichiometric Air Fuel Ratio For Butane The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. What is stoichiometric air fuel ratio? The above formula is for a single. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The amount of air required for combusting. Stoichiometric Air Fuel Ratio For Butane.
From www.slideserve.com
PPT OBJECTIVES PowerPoint Presentation, free download ID4431556 Stoichiometric Air Fuel Ratio For Butane The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. For example, if we have a mixture of. Stoichiometric Air Fuel Ratio For Butane.
From www.numerade.com
SOLVEDA burner receives a mixture of two fuels with mass fraction 40 Stoichiometric Air Fuel Ratio For Butane The above formula is for a single. What is stoichiometric air fuel ratio? For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. The amount of air required for combusting. Stoichiometric Air Fuel Ratio For Butane.
From www.slideserve.com
PPT Stoichiometric Calculations PowerPoint Presentation, free Stoichiometric Air Fuel Ratio For Butane The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. What is stoichiometric air. Stoichiometric Air Fuel Ratio For Butane.
From www.slideserve.com
PPT Unit I PowerPoint Presentation, free download ID3554984 Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The stoichiometric air fuel ratio is the ratio that gives the amount of air required for. Stoichiometric Air Fuel Ratio For Butane.
From workshopfixoharayuko.z21.web.core.windows.net
Stoichiometric Ratio In Engine Stoichiometric Air Fuel Ratio For Butane The above formula is for a single. The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. What is stoichiometric air fuel ratio? The stoichiometric air fuel ratio is the ratio that gives the amount of air required for the complete. Air fuel ratio is defined as the ratio of air and fuel of. Stoichiometric Air Fuel Ratio For Butane.
From www.researchgate.net
Conversion ratios at under‐stoichiometric air‐to‐fuel ratios when CO is Stoichiometric Air Fuel Ratio For Butane For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 31, pt 4 of 5 Combustion Stoichiometric Air Fuel Ratio For Butane When all the fuel is combined with all the free oxygen, typically within a vehicle’s combustion chamber, the reaction is chemically balanced. For example, if we have a mixture of methane and air which has the air fuel ratio of 17.5, it means that. The above formula is for a single. The stoichiometric air fuel ratio is the ratio that. Stoichiometric Air Fuel Ratio For Butane.
From www.coursehero.com
[Solved] Calculate the stoichiometric fuelair ratio on a volume basis Stoichiometric Air Fuel Ratio For Butane Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. What is stoichiometric air fuel ratio? The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. The above formula is for a single. The stoichiometric air fuel ratio is the ratio that gives the amount of. Stoichiometric Air Fuel Ratio For Butane.
From www.youtube.com
Air Fuel Ratio Explained YouTube Stoichiometric Air Fuel Ratio For Butane Air fuel ratio is defined as the ratio of air and fuel of a mixture prepared for combustion. What is stoichiometric air fuel ratio? The amount of air required for combusting a stoichiometric mixture is called stoichiometric or theoretical air. The above formula is for a single. When all the fuel is combined with all the free oxygen, typically within. Stoichiometric Air Fuel Ratio For Butane.