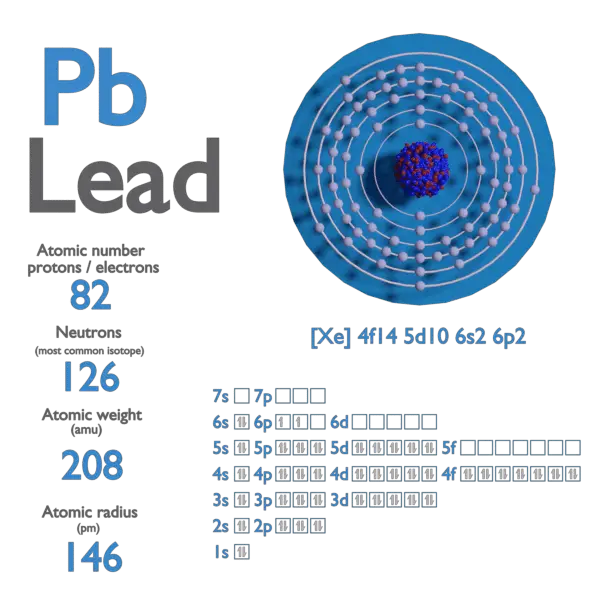
Lead-206 Electrons . 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. Lead occurs in 4 natural isotopes: The lead atom has a. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. In nature, the chemical element lead occurs in the form of 8 different isotopes: Properties and data of the isotope 206 pb.
from ar.inspiredpencil.com
208 pb is the most common isotope, having a natural abundance of approximately 52%. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. Lead occurs in 4 natural isotopes: Properties and data of the isotope 206 pb. In nature, the chemical element lead occurs in the form of 8 different isotopes: 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The lead atom has a. 204 pb, 206 pb, 207 pb and 208 pb.
Lead Atom Electrons
Lead-206 Electrons 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. 204 pb, 206 pb, 207 pb and 208 pb. The lead atom has a. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. In nature, the chemical element lead occurs in the form of 8 different isotopes: Lead occurs in 4 natural isotopes: 208 pb is the most common isotope, having a natural abundance of approximately 52%. Properties and data of the isotope 206 pb. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2.
From www.alamy.com
Atom symbol electron lead illustration hires stock photography and Lead-206 Electrons Properties and data of the isotope 206 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. In nature, the chemical element lead occurs in the form of 8 different isotopes: The lead atom has a. Lead occurs in 4 natural isotopes:. Lead-206 Electrons.
From www.researchgate.net
Uranium238 radioactive chain diagram. The radioactive chain begins Lead-206 Electrons The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. Properties and data of the isotope 206 pb. Lead occurs in 4 natural isotopes: The lead atom has a. 208 pb is the most common isotope, having a natural abundance. Lead-206 Electrons.
From www.numerade.com
SOLVEDHow many protons, neutrons, and electrons are there in (a) ^3 He Lead-206 Electrons 204 pb, 206 pb, 207 pb and 208 pb. The lead atom has a. Properties and data of the isotope 206 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The number of electrons in each of lead's shells is [2,. Lead-206 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-206 Electrons Properties and data of the isotope 206 pb. Lead occurs in 4 natural isotopes: The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. The lead atom has a. 208 pb is the most common isotope, having a natural abundance. Lead-206 Electrons.
From www.numerade.com
SOLVEDWrite the balanced nuclear equation for each of the following Lead-206 Electrons The lead atom has a. In nature, the chemical element lead occurs in the form of 8 different isotopes: Properties and data of the isotope 206 pb. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 208 pb is. Lead-206 Electrons.
From www.buyisotope.com
Pb206 Isotope, Enriched Pb206, Pb206 Metal, Pb206 Oxide Lead-206 Electrons In nature, the chemical element lead occurs in the form of 8 different isotopes: The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 204 pb, 206 pb, 207 pb and 208 pb. The lead atom has a. Lead occurs. Lead-206 Electrons.
From animalia-life.club
Lead Bohr Model Lead-206 Electrons Lead occurs in 4 natural isotopes: In nature, the chemical element lead occurs in the form of 8 different isotopes: 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and. Lead-206 Electrons.
From www.sciencephoto.com
Lead, atomic structure Stock Image C013/1639 Science Photo Library Lead-206 Electrons Lead occurs in 4 natural isotopes: In nature, the chemical element lead occurs in the form of 8 different isotopes: 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The lead atom has a. Properties and data of the isotope 206 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%.. Lead-206 Electrons.
From www.buyisotope.com
Lead206, Lead206 Isotope, Enriched Lead206, Lead206 Metal Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. Lead occurs in 4 natural isotopes: 4 stable nuclides (lead 204, 206, 207, 208) and 4. Lead-206 Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead-206 Electrons 204 pb, 206 pb, 207 pb and 208 pb. The lead atom has a. 208 pb is the most common isotope, having a natural abundance of approximately 52%. In nature, the chemical element lead occurs in the form of 8 different isotopes: Lead occurs in 4 natural isotopes: The number of electrons in each of lead's shells is [2, 8,. Lead-206 Electrons.
From dxovcaane.blob.core.windows.net
Shorthand Electron Configuration For Lead at Perry blog Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. Properties and data of the isotope 206 pb. In nature, the chemical element lead occurs in the form of 8 different isotopes: Lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in each of lead's shells. Lead-206 Electrons.
From www.dreamstime.com
Lead stock illustration. Illustration of atomic, symbol 175796461 Lead-206 Electrons The lead atom has a. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 204 pb, 206 pb, 207 pb and 208 pb. Lead occurs in 4 natural isotopes: Properties and data of the isotope 206 pb. In nature,. Lead-206 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-206 Electrons 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. Lead occurs in 4 natural isotopes: 208 pb is the most common isotope, having a natural abundance of approximately 52%. The lead atom has a. 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in each of lead's shells is [2, 8, 18,. Lead-206 Electrons.
From joifefnuf.blob.core.windows.net
Lead Complete Electron Configuration at Dawn Westbury blog Lead-206 Electrons Properties and data of the isotope 206 pb. 204 pb, 206 pb, 207 pb and 208 pb. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. Lead occurs in 4 natural isotopes: The lead atom has a. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron. Lead-206 Electrons.
From periodictable.com
Isotope data for lead206 in the Periodic Table Lead-206 Electrons Lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. Properties and data of the isotope 206 pb. The lead atom has a. 4 stable. Lead-206 Electrons.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead-206 Electrons 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. 208 pb is the most common isotope, having a natural abundance of approximately 52%. Properties and data of the isotope 206 pb. In nature, the chemical element lead occurs in the form of 8 different isotopes: The lead atom has a. The number of electrons in each. Lead-206 Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. Properties and data of the isotope 206 pb. Lead occurs in 4 natural isotopes: In nature, the chemical element lead occurs in the form of 8 different isotopes: 204 pb, 206 pb, 207 pb and 208 pb. 4 stable nuclides (lead 204, 206, 207, 208) and. Lead-206 Electrons.
From www.bartleby.com
Answered Be sure to answer all parts. Lead206… bartleby Lead-206 Electrons Properties and data of the isotope 206 pb. Lead occurs in 4 natural isotopes: The lead atom has a. 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 208 pb. Lead-206 Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead-206 Electrons 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. 204 pb, 206 pb, 207 pb and 208 pb. In nature, the chemical element lead occurs in the form of 8 different isotopes: 208 pb is the most common isotope, having a natural abundance of approximately 52%. The number of electrons in each of lead's shells is. Lead-206 Electrons.
From ar.inspiredpencil.com
Lead Symbol Lead-206 Electrons The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. The lead atom has a. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. Properties and data of the isotope 206 pb. 204 pb, 206 pb,. Lead-206 Electrons.
From cabinet.matttroy.net
Lead Periodic Table Electrons Matttroy Lead-206 Electrons 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. Lead occurs in 4 natural isotopes: 204 pb, 206 pb, 207 pb and 208 pb. In nature, the chemical element lead occurs in the form of 8 different isotopes: The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its. Lead-206 Electrons.
From material-properties.org
Lead Periodic Table and Atomic Properties Lead-206 Electrons The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 208 pb is the most common isotope, having a natural abundance of approximately 52%. In nature, the chemical element lead occurs in the form of 8 different isotopes: The lead. Lead-206 Electrons.
From pressbooks.bccampus.ca
2.1 Elements and Atoms the Building Blocks of Matter — Anatomy and Lead-206 Electrons 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. Properties and data of the isotope 206 pb.. Lead-206 Electrons.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Art Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The lead atom has a. Lead occurs in 4 natural isotopes: The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe]. Lead-206 Electrons.
From www.youtube.com
CHEMISTRY 101 Writing an Electron Configuration for Lead Using the Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. The lead atom has a. 4 stable. Lead-206 Electrons.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead-206 Electrons The lead atom has a. Lead occurs in 4 natural isotopes: Properties and data of the isotope 206 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. 204 pb, 206 pb, 207 pb and 208 pb. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The number of electrons in. Lead-206 Electrons.
From 3dwarehouse.sketchup.com
Lead 206 Option 2 3D Warehouse Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. Lead occurs in 4 natural isotopes: 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s. Lead-206 Electrons.
From www.buyisotope.com
Lead206, Lead206 Isotope, Enriched Lead206, Lead206 Metal Lead-206 Electrons 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The lead atom has a. 208 pb is the most common isotope, having a natural abundance of approximately 52%. In nature, the chemical element lead occurs in the form of 8 different isotopes: 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in. Lead-206 Electrons.
From periodictable.me
Lead Valence Electrons Lead Valency (Pb) with Dot Diagram Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. In nature, the chemical element lead occurs in the form of 8 different isotopes: The lead atom has a. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s. Lead-206 Electrons.
From www.numerade.com
SOLVEDHow many protons, neutrons, and electrons are present in an atom Lead-206 Electrons Lead occurs in 4 natural isotopes: Properties and data of the isotope 206 pb. The lead atom has a. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. In nature, the chemical element. Lead-206 Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead-206 Electrons 204 pb, 206 pb, 207 pb and 208 pb. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. In nature, the chemical element lead occurs in the form of 8 different isotopes: Properties and data of the isotope 206. Lead-206 Electrons.
From www.alamy.com
Pb Lead, Periodic Table of the Elements, Shell Structure of Lead Lead-206 Electrons Properties and data of the isotope 206 pb. The lead atom has a. 208 pb is the most common isotope, having a natural abundance of approximately 52%. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration. Lead-206 Electrons.
From awesomehome.co
Lead Periodic Table Electrons Awesome Home Lead-206 Electrons The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s 2 6p 2. Properties and data of the isotope 206 pb. In nature, the chemical element lead occurs in the form of 8 different isotopes: Lead occurs in 4 natural isotopes: 4 stable. Lead-206 Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. 4 stable nuclides (lead 204, 206, 207, 208) and 4 radionuclides (lead 210,. Lead occurs in 4 natural isotopes: The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s. Lead-206 Electrons.
From www.alamy.com
Lead atom, with mass and energy levels. Vector illustration Stock Lead-206 Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. In nature, the chemical element lead occurs in the form of 8 different isotopes: The lead atom has a. The number of electrons in each of lead's shells is [2, 8, 18, 32, 18, 4] and its electron configuration is [xe] 4f 14 5d 10 6s. Lead-206 Electrons.