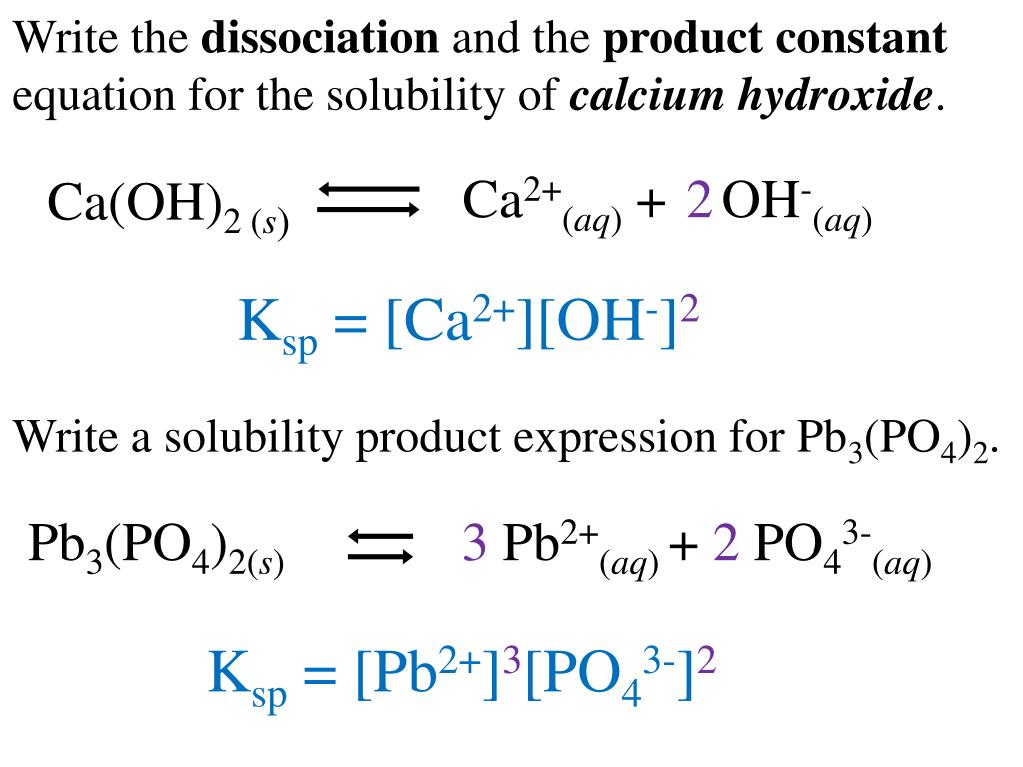
Iron 3 Hydroxide Dissociation Equation . Enter an equation of an ionic chemical equation and press the balance button. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Fe2(so4)3 → 2fe3+ + 3so2− 4. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. It is important to be. An ionic crystal lattice breaks apart when it is dissolved in water. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. The balanced equation will be calculated along with the. Calculate the solubility of iron(ii) hydroxide in a. To dissociate means to take apart. When ionic compounds (compounds made from metals and. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c;
from www.slideserve.com
Fe2(so4)3 → 2fe3+ + 3so2− 4. To dissociate means to take apart. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Calculate the solubility of iron(ii) hydroxide in a. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. When ionic compounds (compounds made from metals and. An ionic crystal lattice breaks apart when it is dissolved in water. It is important to be. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79.
PPT Solubility Equilibria PowerPoint Presentation, free download ID
Iron 3 Hydroxide Dissociation Equation An ionic crystal lattice breaks apart when it is dissolved in water. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. The balanced equation will be calculated along with the. Calculate the solubility of iron(ii) hydroxide in a. An ionic crystal lattice breaks apart when it is dissolved in water. Fe2(so4)3 → 2fe3+ + 3so2− 4. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. To dissociate means to take apart. It is important to be. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Enter an equation of an ionic chemical equation and press the balance button. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; When ionic compounds (compounds made from metals and.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron 3 Hydroxide Dissociation Equation It is important to be. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. To dissociate means to take apart. An ionic crystal lattice breaks apart when it is dissolved in water. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron. Iron 3 Hydroxide Dissociation Equation.
From chemistryguru.com.sg
How to Calculate Solubility in Presence of Common Ion Iron 3 Hydroxide Dissociation Equation Enter an equation of an ionic chemical equation and press the balance button. An ionic crystal lattice breaks apart when it is dissolved in water. Fe2(so4)3 → 2fe3+ + 3so2− 4. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
The reaction of iron(III) solution with sodium hydroxide and ammonia Iron 3 Hydroxide Dissociation Equation Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Enter an equation of an ionic chemical equation and press the balance button. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Fe(OH)3 + HCl = FeCl3 + H2O Iron 3 Hydroxide Dissociation Equation Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Enter an equation of an ionic chemical equation and press the balance button. An ionic crystal lattice breaks apart when it is dissolved in water. Fe2(so4)3 → 2fe3+ + 3so2− 4. When ionic compounds (compounds made from metals and. Iron(ii) hydroxide is only sparingly. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Net ionic equation Complete iron(III) ionic equation chloride Iron 3 Hydroxide Dissociation Equation Enter an equation of an ionic chemical equation and press the balance button. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Calculate the solubility of iron(ii) hydroxide in a. To dissociate means to take apart. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79.. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED The thermal of iron(III) hydroxide to produce Iron 3 Hydroxide Dissociation Equation To dissociate means to take apart. It is important to be. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Enter an equation of an ionic chemical equation and press the balance button. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Iron(ii) hydroxide is. Iron 3 Hydroxide Dissociation Equation.
From www.coursehero.com
[Solved] Need Proper and Clear answer . Iron III hydroxide, which is a Iron 3 Hydroxide Dissociation Equation Fe2(so4)3 → 2fe3+ + 3so2− 4. To dissociate means to take apart. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; An ionic crystal lattice breaks apart when it is dissolved in water. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Enter an equation of an. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Shown below is the solubility diagram for iron hydroxide Fe(OH Iron 3 Hydroxide Dissociation Equation Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Enter an equation of an ionic chemical equation and press the balance button. Fe2(so4)3 → 2fe3+ + 3so2− 4. An ionic crystal lattice breaks apart when it is dissolved in water. The $k_\mathrm{sp}$ of. Iron 3 Hydroxide Dissociation Equation.
From www.sciencephoto.com
Iron (III) hydroxide precipitate Stock Image A500/0413 Science Iron 3 Hydroxide Dissociation Equation When ionic compounds (compounds made from metals and. Fe2(so4)3 → 2fe3+ + 3so2− 4. It is important to be. The balanced equation will be calculated along with the. An ionic crystal lattice breaks apart when it is dissolved in water. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as. Iron 3 Hydroxide Dissociation Equation.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Iron 3 Hydroxide Dissociation Equation Calculate the solubility of iron(ii) hydroxide in a. It is important to be. An ionic crystal lattice breaks apart when it is dissolved in water. When ionic compounds (compounds made from metals and. The balanced equation will be calculated along with the. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Write out the reaction that occurs when lithium phosphate Iron 3 Hydroxide Dissociation Equation Fe2(so4)3 → 2fe3+ + 3so2− 4. To dissociate means to take apart. The balanced equation will be calculated along with the. When ionic compounds (compounds made from metals and. It is important to be. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source,. Iron 3 Hydroxide Dissociation Equation.
From www.toppr.com
Iron III Hydroxide Formula Definition, Concepts and Examples Iron 3 Hydroxide Dissociation Equation Enter an equation of an ionic chemical equation and press the balance button. To dissociate means to take apart. The balanced equation will be calculated along with the. It is important to be. An ionic crystal lattice breaks apart when it is dissolved in water. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Fe2(so4)3. Iron 3 Hydroxide Dissociation Equation.
From www.chegg.com
Solved Consider the chemical reaction that takes place Iron 3 Hydroxide Dissociation Equation To dissociate means to take apart. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. Calculate the solubility of iron(ii) hydroxide in a. Enter an equation of an ionic chemical equation and press the balance button. Dissociation is the separation of ions. Iron 3 Hydroxide Dissociation Equation.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Iron 3 Hydroxide Dissociation Equation Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Fe2(so4)3 → 2fe3+ + 3so2− 4. The balanced equation will be calculated along. Iron 3 Hydroxide Dissociation Equation.
From sciencephotogallery.com
Iron (iii) Hydroxide Precipitation by Science Photo Library Iron 3 Hydroxide Dissociation Equation Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. The balanced equation will be calculated along with the. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. When ionic compounds (compounds made from metals and. Enter an equation of an ionic chemical equation and press. Iron 3 Hydroxide Dissociation Equation.
From studylibconcents.z21.web.core.windows.net
How To Write Dissociation Equations Iron 3 Hydroxide Dissociation Equation The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. To dissociate means to take apart. Fe2(so4)3 → 2fe3+ + 3so2− 4. An ionic crystal lattice breaks apart when it is dissolved in water. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Solubility rules are. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
Dissociation of Ionic Compounds YouTube Iron 3 Hydroxide Dissociation Equation Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. The balanced equation will be calculated along with the. Fe2(so4)3 → 2fe3+ + 3so2− 4. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Iron(III) sulfate is made in industry by the neutralization Iron 3 Hydroxide Dissociation Equation Calculate the solubility of iron(ii) hydroxide in a. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. To dissociate means to take apart. Enter an equation of an. Iron 3 Hydroxide Dissociation Equation.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Iron 3 Hydroxide Dissociation Equation The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. To dissociate means to take apart. Dissociation is the separation of ions that. Iron 3 Hydroxide Dissociation Equation.
From learnaboutpharma.com
Dissociation constant Definition, Explanation, Formula, Example, and Iron 3 Hydroxide Dissociation Equation The balanced equation will be calculated along with the. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. Enter an equation of an ionic chemical equation and press the balance. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
What mass of iron(III) hydroxide precipitate can be produced by Iron 3 Hydroxide Dissociation Equation Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Calculate the solubility of iron(ii) hydroxide in a. Dissociation is the separation of. Iron 3 Hydroxide Dissociation Equation.
From www.chegg.com
Solved 2. Iron (III) chloride dissolves in water forming Iron 3 Hydroxide Dissociation Equation Enter an equation of an ionic chemical equation and press the balance button. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. It is important to be. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. When ionic compounds (compounds made from metals and. Iron. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
Gas stoichiometry of iron(III) hydroxide YouTube Iron 3 Hydroxide Dissociation Equation Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. An ionic crystal lattice breaks apart when it is dissolved in water. Iron(ii) hydroxide is only sparingly. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
How to Write the Formula for Iron (III) hydroxide YouTube Iron 3 Hydroxide Dissociation Equation Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Calculate the solubility of iron(ii) hydroxide in a. It is important to be. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. To dissociate. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
How to find the percent composition of Fe(OH)3 (Iron (III) Hydroxide Iron 3 Hydroxide Dissociation Equation Enter an equation of an ionic chemical equation and press the balance button. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. It is important to be. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
Equation for Fe(OH)3 + H2O Iron (III) hydroxide + Water YouTube Iron 3 Hydroxide Dissociation Equation The balanced equation will be calculated along with the. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Calculate the solubility of iron(ii) hydroxide in a. Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or. Iron 3 Hydroxide Dissociation Equation.
From kunduz.com
[ANSWERED] the balanced equation that descri dissoci... Physical Iron 3 Hydroxide Dissociation Equation To dissociate means to take apart. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Calculate the solubility of iron(ii) hydroxide in a. When ionic compounds (compounds made from metals and. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. It is important to be.. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVEDIron(II) hydrogen phosphite, FeHPO3, is oxidized by hypochlorite Iron 3 Hydroxide Dissociation Equation To dissociate means to take apart. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Calculate the solubility of iron(ii) hydroxide in a. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Iron(ii) hydroxide is only sparingly soluble in water at 25 o. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED A student writes the following incorrect chemical equation for Iron 3 Hydroxide Dissociation Equation Calculate the solubility of iron(ii) hydroxide in a. It is important to be. When ionic compounds (compounds made from metals and. The balanced equation will be calculated along with the. Fe2(so4)3 → 2fe3+ + 3so2− 4. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Iron(ii) hydroxide is only sparingly soluble in water. Iron 3 Hydroxide Dissociation Equation.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron 3 Hydroxide Dissociation Equation The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. An ionic crystal lattice breaks apart when it is dissolved in water. It is important to be. Calculate the solubility of iron(ii) hydroxide in a. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable. Iron 3 Hydroxide Dissociation Equation.
From www.numerade.com
SOLVED Write balanced chemical equations for the following reactions Iron 3 Hydroxide Dissociation Equation The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. When ionic compounds (compounds made from metals and. Fe2(so4)3 → 2fe3+ + 3so2− 4. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Iron. Iron 3 Hydroxide Dissociation Equation.
From slideplayer.com
Balancing Equations and Types of Reactions ppt download Iron 3 Hydroxide Dissociation Equation Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; Iron (iii) hydroxide is most commonly synthesized in the laboratory by adding a solution of iron (iii) salt, such as iron (iii) chloride or nitrate, to a. When ionic compounds (compounds made from metals. Iron 3 Hydroxide Dissociation Equation.
From www.slideserve.com
PPT Classifying Reaction PowerPoint Presentation, free download ID Iron 3 Hydroxide Dissociation Equation Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Fe2(so4)3 → 2fe3+ + 3so2− 4. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the. Iron 3 Hydroxide Dissociation Equation.
From www.youtube.com
How to Balance Fe(OH)3 + H2SO4 = Fe2(SO4)3 + H2O YouTube Iron 3 Hydroxide Dissociation Equation Calculate the solubility of iron(ii) hydroxide in a. Fe2(so4)3 → 2fe3+ + 3so2− 4. The balanced equation will be calculated along with the. The $k_\mathrm{sp}$ of $\ce{fe(oh)3}$ is varied from source to source, but a reliable source gives the value of $2.79. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. It is important to. Iron 3 Hydroxide Dissociation Equation.
From www.shutterstock.com
Iron Iii Hydroxide Formula Handwritten Chemical Stock Illustration Iron 3 Hydroxide Dissociation Equation When ionic compounds (compounds made from metals and. To dissociate means to take apart. Iron(ii) hydroxide is only sparingly soluble in water at 25 o c; The balanced equation will be calculated along with the. Fe2(so4)3 → 2fe3+ + 3so2− 4. An ionic crystal lattice breaks apart when it is dissolved in water. Calculate the solubility of iron(ii) hydroxide in. Iron 3 Hydroxide Dissociation Equation.