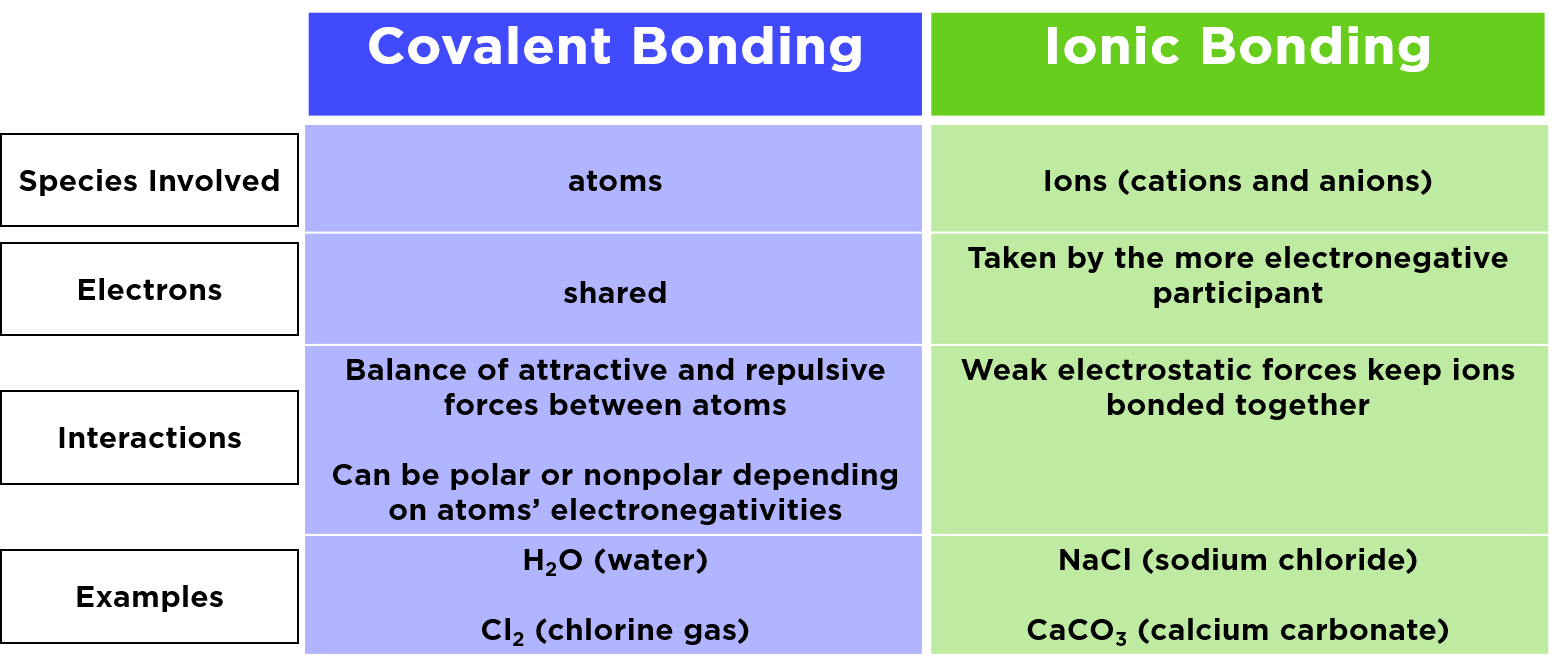
Bri Ionic Or Covalent . Here is an explanation of the difference between ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Iodine monobromide is an interhalogen compound with the formula ibr. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. This works best when the atoms in question have similar. It is a dark red solid that melts near room temperature.
from www.expii.com
A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. It is a dark red solid that melts near room temperature. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. Here is an explanation of the difference between ionic. This works best when the atoms in question have similar. Iodine monobromide is an interhalogen compound with the formula ibr. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements.
Ionic Bonding (Biology) — Definition & Role Expii
Bri Ionic Or Covalent It is a dark red solid that melts near room temperature. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. This works best when the atoms in question have similar. It is a dark red solid that melts near room temperature. Iodine monobromide is an interhalogen compound with the formula ibr. Here is an explanation of the difference between ionic. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Bri Ionic Or Covalent This works best when the atoms in question have similar. It is a dark red solid that melts near room temperature. Here is an explanation of the difference between ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Iodine monobromide is an interhalogen compound with the formula ibr.. Bri Ionic Or Covalent.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo Bri Ionic Or Covalent Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. It is a dark red solid that melts near room temperature. Here is an explanation of the difference between ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. This works best when. Bri Ionic Or Covalent.
From giovannigokesalas.blogspot.com
Difference Between Ionic and Covalent Bonds Bri Ionic Or Covalent Here is an explanation of the difference between ionic. It is a dark red solid that melts near room temperature. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. This works best when the atoms in question have similar. The main difference between ionic and covalent bonds is how equally the electrons. Bri Ionic Or Covalent.
From www.slideserve.com
PPT Naming Ionic Compounds and Covalent Molecules PowerPoint Bri Ionic Or Covalent A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Here is an explanation of the difference between ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The bond polarity between two atoms can be estimated if. Bri Ionic Or Covalent.
From www.youtube.com
Differentiating between ionic vs covalent compounds YouTube Bri Ionic Or Covalent The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Here is an explanation of the difference between ionic. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Iodine monobromide is an interhalogen compound with the formula ibr. The main difference. Bri Ionic Or Covalent.
From derekcarrsavvy-chemist.blogspot.co.uk
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Bri Ionic Or Covalent It is a dark red solid that melts near room temperature. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. Here is an explanation of the difference between ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The bond polarity between. Bri Ionic Or Covalent.
From pediaa.com
Difference Between Ionic Covalent and Metallic Bonds Definition Bri Ionic Or Covalent It is a dark red solid that melts near room temperature. Iodine monobromide is an interhalogen compound with the formula ibr. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The. Bri Ionic Or Covalent.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary Bri Ionic Or Covalent It is a dark red solid that melts near room temperature. This works best when the atoms in question have similar. Here is an explanation of the difference between ionic. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Covalent bonds are formed when nonmetals form compounds with each other by sharing. Bri Ionic Or Covalent.
From www.slideserve.com
PPT Chemistry Test Review PowerPoint Presentation, free download Bri Ionic Or Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Iodine monobromide is an interhalogen compound with the formula ibr. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. It is a dark red solid that melts near room temperature. A bond in. Bri Ionic Or Covalent.
From pediaa.com
Difference Between Covalent and Ionic Bonds Bri Ionic Or Covalent Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. Iodine monobromide is an interhalogen compound with the formula ibr. This works best when the atoms in question have similar. Here is an explanation of the difference between ionic. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1. Bri Ionic Or Covalent.
From www.logiota.com
Ionic and Covalent Bond Chemical Bonding Physical Chemistry Bri Ionic Or Covalent Here is an explanation of the difference between ionic. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Iodine monobromide is an interhalogen compound with the formula ibr. It is a dark red. Bri Ionic Or Covalent.
From clarence-well-duffy.blogspot.com
Explain the Major Differences Between Covalent and Ionic Bonding Bri Ionic Or Covalent This works best when the atoms in question have similar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Here is an explanation of the difference between ionic. Covalent bonds are. Bri Ionic Or Covalent.
From www.youtube.com
Is Br2 Ionic or Covalent / Molecular? YouTube Bri Ionic Or Covalent It is a dark red solid that melts near room temperature. Here is an explanation of the difference between ionic. Iodine monobromide is an interhalogen compound with the formula ibr. This works best when the atoms in question have similar. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Covalent bonds are. Bri Ionic Or Covalent.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Bri Ionic Or Covalent The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. This works best when the atoms in question have similar. Iodine monobromide is an interhalogen compound with the formula ibr. Here is an. Bri Ionic Or Covalent.
From www.slideserve.com
PPT Chemical Bonds (Ionic and Covalent Bonds) Part 1 PowerPoint Bri Ionic Or Covalent Iodine monobromide is an interhalogen compound with the formula ibr. This works best when the atoms in question have similar. It is a dark red solid that melts near room temperature. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The main difference between ionic and covalent bonds is how equally the. Bri Ionic Or Covalent.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart Bri Ionic Or Covalent Here is an explanation of the difference between ionic. This works best when the atoms in question have similar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. It is a. Bri Ionic Or Covalent.
From educational.my.id
Ionic And Covalent Bonds Worksheet Educational.my.id Bri Ionic Or Covalent Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. It is a dark red solid that melts near room temperature. This works best when the atoms in question have similar. Here is an explanation of the difference between ionic. Iodine monobromide is an interhalogen compound with the formula ibr. A bond in which. Bri Ionic Or Covalent.
From www.slideserve.com
PPT Chemical Bonding and Nomenclature PowerPoint Presentation, free Bri Ionic Or Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Iodine monobromide is an interhalogen compound with the formula ibr. It is a dark red solid that melts near room temperature. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. This works. Bri Ionic Or Covalent.
From www.masterorganicchemistry.com
Ionic and Covalent Bonding Master Organic Chemistry Bri Ionic Or Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. It is a dark red solid that melts near room temperature. Iodine monobromide is an interhalogen compound with the formula ibr. Here is an explanation of the difference between ionic. The bond polarity between two atoms can be estimated if. Bri Ionic Or Covalent.
From slidetodoc.com
PART I Bonding Basics Bonding is an example Bri Ionic Or Covalent This works best when the atoms in question have similar. Iodine monobromide is an interhalogen compound with the formula ibr. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Here is an. Bri Ionic Or Covalent.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds Bri Ionic Or Covalent This works best when the atoms in question have similar. Here is an explanation of the difference between ionic. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. It is a dark red solid that melts. Bri Ionic Or Covalent.
From sciencenotes.org
Ionic vs Covalent Bonds Bri Ionic Or Covalent The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Here is an explanation of the difference between ionic. Iodine monobromide is an interhalogen compound with the formula ibr. It is a dark red solid that melts near room temperature. Covalent bonds are formed when nonmetals form compounds with each other by sharing. Bri Ionic Or Covalent.
From infogram.com
Covalent Bonds .VS. Ionic Bonds Infogram Bri Ionic Or Covalent Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Here is an explanation of the difference between ionic. Iodine monobromide is an interhalogen compound with the formula ibr. The main difference between. Bri Ionic Or Covalent.
From www.youtube.com
How to tell if Ionic Bond or Covalent Bond YouTube Bri Ionic Or Covalent A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. The bond polarity. Bri Ionic Or Covalent.
From www.currentschoolnews.com
Major Differences Between Ionic and Covalent Compounds People Ignore Bri Ionic Or Covalent Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Iodine monobromide is an interhalogen compound with the formula ibr. The main difference between ionic and covalent bonds is how equally the electrons. Bri Ionic Or Covalent.
From blogs.glowscotland.org.uk
Review Comparing Ionic/ Covalent Compounds S3 Chemistry Consolidation Bri Ionic Or Covalent Here is an explanation of the difference between ionic. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. It is a dark red solid that melts near room temperature. This works. Bri Ionic Or Covalent.
From www.yourdictionary.com
Main Differences Between Ionic and Covalent Bonds YourDictionary Bri Ionic Or Covalent The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. It is a dark red solid that melts near room temperature. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. Iodine monobromide is an interhalogen compound with the formula ibr. A bond in which the electronegativity. Bri Ionic Or Covalent.
From mungfali.com
Ionic And Covalent Bonds Venn Diagram Bri Ionic Or Covalent The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. It is a dark red solid that melts near room temperature. This works best when the atoms in question have similar. Here is an. Bri Ionic Or Covalent.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Bri Ionic Or Covalent It is a dark red solid that melts near room temperature. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. This works best when the atoms in question have similar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Iodine. Bri Ionic Or Covalent.
From slideplayer.com
Overview Bonding, Structure and the properties of matter ppt download Bri Ionic Or Covalent Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. This works best when the atoms in question have similar. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called. Bri Ionic Or Covalent.
From www.showme.com
ShowMe ionic bonds covalent bonds Bri Ionic Or Covalent Iodine monobromide is an interhalogen compound with the formula ibr. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. The main difference between ionic and covalent bonds is how equally the electrons. Bri Ionic Or Covalent.
From www.slideserve.com
PPT Naming Covalent/ Molecular Compounds PowerPoint Presentation Bri Ionic Or Covalent Here is an explanation of the difference between ionic. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Iodine monobromide is an interhalogen compound with the formula ibr. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. This works best when the atoms in question. Bri Ionic Or Covalent.
From electronegativityisprettyawesome.weebly.com
What are the differences between ionic, polar covalent, nonpolar Bri Ionic Or Covalent The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. This works best when the atoms in. Bri Ionic Or Covalent.
From www.slideserve.com
PPT Chemical Names and Formulas PowerPoint Presentation, free Bri Ionic Or Covalent Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. Iodine monobromide is an interhalogen compound with the formula ibr. This works best when the atoms in question have similar. Here is an explanation of the difference between ionic. It is a dark red solid that melts near room temperature. A bond in which. Bri Ionic Or Covalent.
From www.slideserve.com
PPT Metallic Bonds & Ionic Bonds PowerPoint Presentation, free Bri Ionic Or Covalent Iodine monobromide is an interhalogen compound with the formula ibr. Covalent bonds are formed when nonmetals form compounds with each other by sharing electrons between them. Here is an explanation of the difference between ionic. It is a dark red solid that melts near room temperature. The main difference between ionic and covalent bonds is how equally the electrons are. Bri Ionic Or Covalent.