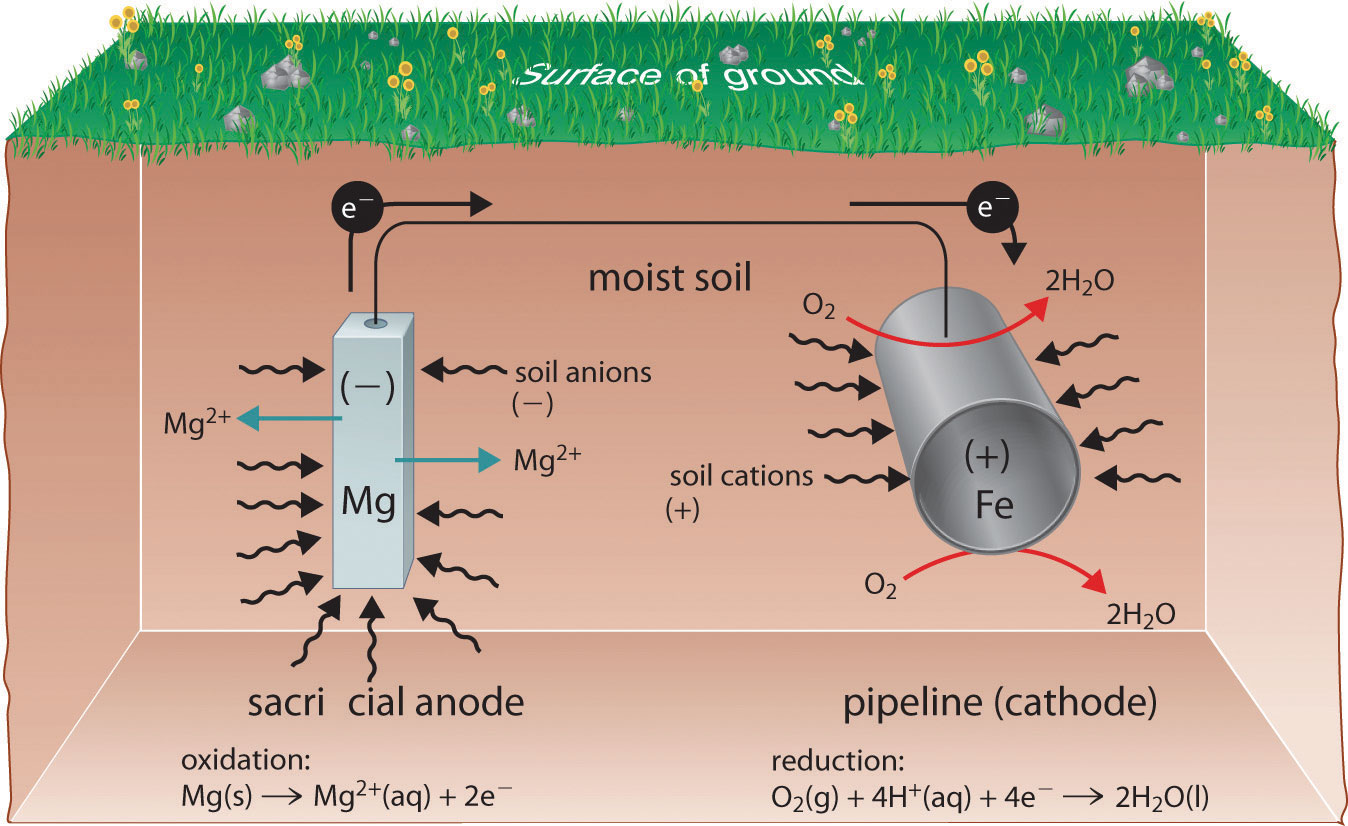
How Does Sacrificial Protection Work . Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. one type of cathodic protection system is the sacrificial anode. sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. The anode is made from a metal alloy with a more active voltage (more negative. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes.
from socratic.org
sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. one type of cathodic protection system is the sacrificial anode. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. The anode is made from a metal alloy with a more active voltage (more negative. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams.
Question 95d51 Socratic
How Does Sacrificial Protection Work sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. The anode is made from a metal alloy with a more active voltage (more negative. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. one type of cathodic protection system is the sacrificial anode. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes.
From www.researchgate.net
Sacrificial anode system. Download Scientific Diagram How Does Sacrificial Protection Work as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. one type of cathodic protection system is the sacrificial anode. The anode is made from a metal alloy with a more active voltage (more negative. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes. How Does Sacrificial Protection Work.
From instrumentationtools.com
Types of Cathodic Protection for Pipeline Protection How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. The anode is made from a metal alloy with a more active voltage (more negative. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. as mentioned previously, cathodic protection works by intentionally forming a. How Does Sacrificial Protection Work.
From www.corrosionpedia.com
Galvanic Cathodic Protection How Does Sacrificial Protection Work sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. sacrificial protection is a corrosion protection method in which a more. How Does Sacrificial Protection Work.
From www.desperatesailors.com
How Do Sacrificial Anodes Work? Find Out Everything Here How Does Sacrificial Protection Work revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other. How Does Sacrificial Protection Work.
From www.vrogue.co
Sacp Sacrificial Anode Cathodic Protection vrogue.co How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. . How Does Sacrificial Protection Work.
From www.slideserve.com
PPT Corrosion, Rusting and How to Fight it. PowerPoint Presentation, free download ID1747651 How Does Sacrificial Protection Work revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. one type of cathodic protection system is the sacrificial anode. sacrificial anodes work by oxidizing more quickly than the. How Does Sacrificial Protection Work.
From www.researchgate.net
Schematic showing cathodic protection methods using sacrificial anode... Download Scientific How Does Sacrificial Protection Work sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. The anode is made from a metal alloy with a more active voltage (more negative. sacrificial anodes work by oxidizing more quickly than the metal it. How Does Sacrificial Protection Work.
From freyssinet.co.uk
Cathodic Protection How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. The anode is. How Does Sacrificial Protection Work.
From www.youtube.com
Zinc for Galvanization vs Sacrificial protection IGCSE Chemistry YouTube How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. The anode is made from a metal alloy with a more active voltage (more negative. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. a sacrificial metal is a metal used as a sacrificial. How Does Sacrificial Protection Work.
From www.vlevr.otds.co.uk
Sacrificial Anode Cathodic Protection (SACP) How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. The anode is made from a metal alloy with a more active voltage (more negative. sacrificial anodes work by oxidizing more quickly than the metal. How Does Sacrificial Protection Work.
From galvanizing.org.uk
Barrier and sacrificial protection How Does Sacrificial Protection Work a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with. How Does Sacrificial Protection Work.
From www.slideserve.com
PPT Extraction of metals PowerPoint Presentation ID208800 How Does Sacrificial Protection Work sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. one type of cathodic protection system is the sacrificial anode. The anode is made from a metal alloy. How Does Sacrificial Protection Work.
From www.researchgate.net
Schematic showing cathodic protection methods using sacrificial anode... Download Scientific How Does Sacrificial Protection Work as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. one type of cathodic protection system is the sacrificial anode. Several different forms of cathode protection are forming alloys,. How Does Sacrificial Protection Work.
From www.researchgate.net
7) Sacrificial Anode Cathodic Protection (SACP) Download Scientific Diagram How Does Sacrificial Protection Work Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. sacrificial protection is. How Does Sacrificial Protection Work.
From www.tdk.com
How We Prevent Rust with Cathodic Protection and Sacrificial Anodes|The Wonders of How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts. How Does Sacrificial Protection Work.
From www.cathodicprotection101.com
Cathodic Protection 101 How Does Sacrificial Protection Work as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a. How Does Sacrificial Protection Work.
From engineercalcs.com
Learn A Real World Cathodic Protection Calculation Engineer Calcs How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. The anode is made from a metal alloy with a more active voltage (more negative. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the. How Does Sacrificial Protection Work.
From www.marineinsight.com
Understanding Sacrificial Anodes on Ship How Does Sacrificial Protection Work The anode is made from a metal alloy with a more active voltage (more negative. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts. How Does Sacrificial Protection Work.
From corrosion-group.com
Cathodic corrosion protection with sacrificial anodes, providing corrosion protection How Does Sacrificial Protection Work sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. as mentioned previously, cathodic protection works. How Does Sacrificial Protection Work.
From www.differencebetween.com
Difference Between Cathodic Protection and Sacrificial Protection Compare the Difference How Does Sacrificial Protection Work revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. a sacrificial metal is a metal used as a sacrificial anode. How Does Sacrificial Protection Work.
From maritimepage.com
Sacrificial Anodes A Guide To Marine Corrosion Protection How Does Sacrificial Protection Work Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. one type of cathodic protection system is the sacrificial anode. The anode is made from a metal alloy with a more active voltage (more negative. a sacrificial. How Does Sacrificial Protection Work.
From www.vrogue.co
How Does Cathodic Protection Work For Ships vrogue.co How Does Sacrificial Protection Work The anode is made from a metal alloy with a more active voltage (more negative. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus,. How Does Sacrificial Protection Work.
From www.slideshare.net
Corrosion, standard grade chemistry How Does Sacrificial Protection Work as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. sacrificial protection is. How Does Sacrificial Protection Work.
From www.researchgate.net
Schematic of Sacrificial Anode Cathodic Protection Method 1.4.2. The... Download Scientific How Does Sacrificial Protection Work as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. sacrificial protection is a corrosion. How Does Sacrificial Protection Work.
From bbsteelinternationnal.blogspot.com
How do sacrificial anodes work in the corrosion protection process? (แอโนดเสียสละทำงานอย่างไรใน How Does Sacrificial Protection Work as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other. How Does Sacrificial Protection Work.
From glossary.periodni.com
Sacrificial protection Chemistry Dictionary & Glossary How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. The anode is made from a metal alloy with a more active. How Does Sacrificial Protection Work.
From www.youtube.com
Cathodic Protection Galvanic / Sacrificial YouTube How Does Sacrificial Protection Work a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. one type of. How Does Sacrificial Protection Work.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free download ID6645971 How Does Sacrificial Protection Work sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with the electrolytes. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. one type of cathodic. How Does Sacrificial Protection Work.
From www.vrogue.co
Cathodic Protection With Sacrificial Anodes Providing vrogue.co How Does Sacrificial Protection Work Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. The anode is made from a. How Does Sacrificial Protection Work.
From www.researchgate.net
7) Sacrificial Anode Cathodic Protection (SACP) Download Scientific Diagram How Does Sacrificial Protection Work The anode is made from a metal alloy with a more active voltage (more negative. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. one type of cathodic protection system is the sacrificial anode. sacrificial protection is a corrosion protection method in which a. How Does Sacrificial Protection Work.
From www.tes.com
Sacrificial Protection (IGCSE/GCSE) Teaching Resources How Does Sacrificial Protection Work sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. The anode is made from a metal alloy with a more active voltage (more negative. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. revision notes on galvanising & sacrificial protection for the cie igcse. How Does Sacrificial Protection Work.
From www.youtube.com
Sacrificial Protection Chemical Reaction and Equation Chemistry Class 10 YouTube How Does Sacrificial Protection Work revision notes on galvanising & sacrificial protection for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with. How Does Sacrificial Protection Work.
From www.youtube.com
Cathodic protection sistem Sacrificial Anode ( SACP ) YouTube How Does Sacrificial Protection Work one type of cathodic protection system is the sacrificial anode. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. sacrificial protection is a corrosion protection method in which a more electrochemically active metal is. sacrificial. How Does Sacrificial Protection Work.
From socratic.org
Question 95d51 Socratic How Does Sacrificial Protection Work a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. one type of cathodic protection system is the sacrificial anode. as mentioned previously, cathodic protection works by intentionally forming a galvanic cell with another sacrificial metal. sacrificial protection is a corrosion protection method in. How Does Sacrificial Protection Work.
From courses.lumenlearning.com
Corrosion Chemistry How Does Sacrificial Protection Work Several different forms of cathode protection are forming alloys, plating, and galvanizing the metal. a sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from. sacrificial anodes work by oxidizing more quickly than the metal it is protecting, being consumed completely before the other metal reacts with. How Does Sacrificial Protection Work.