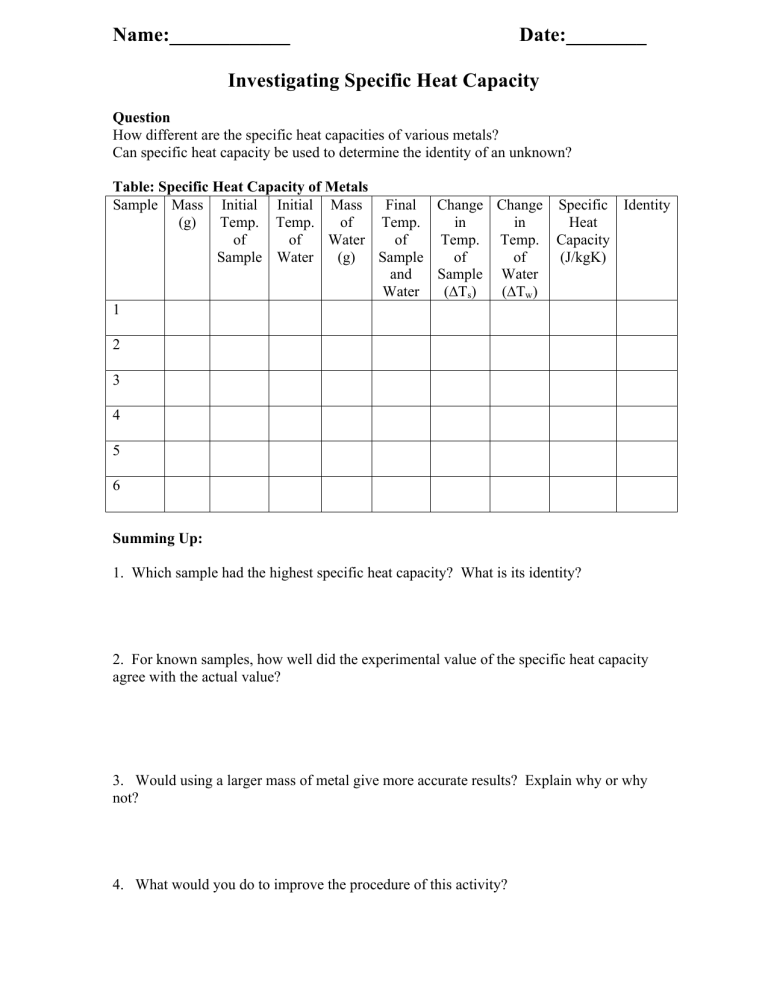
Calorimetry Lab Summary . For example, when an exothermic. You use an aluminum vessel as a calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The first part of the lab session is devoted to evaluating ccal of your calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device used to determine heat flow during a chemical or physical change. A doubled styrofoam cup fitted with a cover in. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The solutions that you are studying should be placed in the in‐.
from studylib.net
Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The solutions that you are studying should be placed in the in‐. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A doubled styrofoam cup fitted with a cover in. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The first part of the lab session is devoted to evaluating ccal of your calorimeter. A calorimeter is a device used to determine heat flow during a chemical or physical change.
calorimetry lab
Calorimetry Lab Summary Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The first part of the lab session is devoted to evaluating ccal of your calorimeter. A calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The solutions that you are studying should be placed in the in‐. A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. For example, when an exothermic. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. You use an aluminum vessel as a calorimeter.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimetry Lab Summary For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an. Calorimetry Lab Summary.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimetry Lab Summary The solutions that you are studying should be placed in the in‐. A doubled styrofoam cup fitted with a cover in. You use an aluminum vessel as a calorimeter. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the amount of. Calorimetry Lab Summary.
From www.scribd.com
Calorimetry Lab Explanation Chemistry Physical Sciences Free 30 Calorimetry Lab Summary A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The solutions that you are studying should be placed in the in‐. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of. Calorimetry Lab Summary.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Calorimetry Lab Summary A calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The solutions that you are studying should be placed in the in‐. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat. Calorimetry Lab Summary.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimetry Lab Summary Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A doubled styrofoam cup fitted with a cover in. For example, when an exothermic. The first part of the lab session is devoted to evaluating ccal of your calorimeter. You use an aluminum vessel as a calorimeter. The solutions that. Calorimetry Lab Summary.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Lab Summary Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A doubled styrofoam cup fitted with a cover in. You use an aluminum vessel as a calorimeter. For example, when an exothermic. The first part of the lab session is devoted to. Calorimetry Lab Summary.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Lab Summary A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The solutions that you are studying should be placed in the in‐. A calorimeter is a device used to measure the amount of heat involved in. Calorimetry Lab Summary.
From studylib.net
Lab 33 calorimetry lab Calorimetry Lab Summary For example, when an exothermic reaction occurs in solution in a calorimeter, the. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device used to determine heat flow during a chemical or physical change. The first part of the lab session is. Calorimetry Lab Summary.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimetry Lab Summary For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to determine heat flow during a chemical or physical change. For example, when an exothermic. You use an aluminum vessel as a calorimeter. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances. Calorimetry Lab Summary.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Dato lob Sec Calorimetry Lab Summary For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. You use an aluminum vessel as a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The solutions that you are studying should be placed in the in‐. In. Calorimetry Lab Summary.
From www.studocu.com
Online Calorimetry Labs Calorimetry Lab Report Sheet Name Quiz is Calorimetry Lab Summary A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. You use an aluminum vessel as a calorimeter. The solutions that you are studying should be placed in the in‐. A doubled styrofoam cup fitted with a cover in. In calorimetry, an insulated reaction container, called a calorimeter, is filled with. Calorimetry Lab Summary.
From www.studocu.com
Formal Lab Report Calorimetry Experiment 25 CALORIMETRY Abstract Calorimetry Lab Summary A calorimeter is a device used to determine heat flow during a chemical or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In calorimetry, an insulated reaction container, called a calorimeter, is. Calorimetry Lab Summary.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimetry Lab Summary For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The first part of the lab session is devoted to evaluating ccal of your calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a. Calorimetry Lab Summary.
From www.studocu.com
Calorimetry Lab Calorimetry Lab Data and Results Table 1 Calorimetry Lab Summary Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is. Calorimetry Lab Summary.
From www.studypool.com
SOLUTION Calorimetry Lab Studypool Calorimetry Lab Summary For example, when an exothermic. You use an aluminum vessel as a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The solutions that you are studying should be placed in the in‐. The. Calorimetry Lab Summary.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Lab Summary The solutions that you are studying should be placed in the in‐. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to determine heat flow during a chemical or physical. Calorimetry Lab Summary.
From studylib.net
06.03 Calorimetry Lab Report Calorimetry Lab Summary For example, when an exothermic. The solutions that you are studying should be placed in the in‐. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures. Calorimetry Lab Summary.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Lab Summary For example, when an exothermic reaction occurs in solution in a calorimeter, the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In calorimetry, an insulated reaction container, called a. Calorimetry Lab Summary.
From www.studocu.com
7. Calorimetry Lab COPY Experiment 6. (1 week) (LCA) Calorimetry Calorimetry Lab Summary Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The solutions that you are studying should be placed in the in‐. The first part of the lab session is devoted to evaluating ccal of your calorimeter. A doubled styrofoam cup fitted. Calorimetry Lab Summary.
From www.studocu.com
Lab 6 Calorimetry Wu. Name Partner Student No Student No Lab Calorimetry Lab Summary The solutions that you are studying should be placed in the in‐. For example, when an exothermic. You use an aluminum vessel as a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A. Calorimetry Lab Summary.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Calorimetry Lab Summary A calorimeter is a device used to determine heat flow during a chemical or physical change. A doubled styrofoam cup fitted with a cover in. The first part of the lab session is devoted to evaluating ccal of your calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry. Calorimetry Lab Summary.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Calorimetry Lab Summary A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. The solutions that you are studying should be placed in the in‐. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Abstract the main purpose of the calorimetry experiment is. Calorimetry Lab Summary.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Lab Summary For example, when an exothermic reaction occurs in solution in a calorimeter, the. The first part of the lab session is devoted to evaluating ccal of your calorimeter. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A calorimeter is a device used to measure the amount of heat. Calorimetry Lab Summary.
From users.highland.edu
Calorimetry Calorimetry Lab Summary A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to determine heat flow during a chemical or physical change. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. The solutions that you are studying should be placed in the in‐.. Calorimetry Lab Summary.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Summary A doubled styrofoam cup fitted with a cover in. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. The first part of the lab session is devoted to evaluating ccal of your calorimeter. A calorimeter is a device used to determine heat flow during a chemical. Calorimetry Lab Summary.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Summary In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. You use an aluminum vessel as a calorimeter. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry measures enthalpy changes during chemical processes, where the. Calorimetry Lab Summary.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Lab Summary A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A doubled styrofoam cup fitted with a cover in. In calorimetry, an insulated reaction container, called a calorimeter, is filled with. Calorimetry Lab Summary.
From studylib.net
calorimetry lab Calorimetry Lab Summary A calorimeter is a device used to determine heat flow during a chemical or physical change. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The solutions that you are studying should be placed in the in‐. In calorimetry, an insulated reaction container, called a calorimeter, is filled with. Calorimetry Lab Summary.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Lab Summary A doubled styrofoam cup fitted with a cover in. The solutions that you are studying should be placed in the in‐. The first part of the lab session is devoted to evaluating ccal of your calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A. Calorimetry Lab Summary.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Calorimetry Lab Summary In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The solutions that you. Calorimetry Lab Summary.
From www.studocu.com
Experiment 25 Calorimetry Prelaboratory Assignment CHEM 005 Studocu Calorimetry Lab Summary The first part of the lab session is devoted to evaluating ccal of your calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Abstract the main purpose of the calorimetry. Calorimetry Lab Summary.
From browsegrades.net
Get Great Study Materials and Great Services Calorimetry Lab Summary For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A calorimeter is a device used to measure. Calorimetry Lab Summary.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Lab Summary Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A calorimeter is a device used to determine heat flow during a chemical or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The first part of the. Calorimetry Lab Summary.
From studylib.net
Student instructions and lab report Calorimetry Lab Summary A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The. Calorimetry Lab Summary.
From www.studocu.com
Calorimetry Lab Report Calorimetry Lab Report Introduction Calorimetry Lab Summary In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to determine heat. Calorimetry Lab Summary.