Zinc Sulphate Electrons . [4] specific reactions include the reaction of the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Connecting the copper electrode to the zinc electrode. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. This phenomena occurs because copper metal is. The positive sign of the copper e° shows that it releases electrons less readily. Then a zinc electrode is placed in the zinc sulfate solution. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A zinc sulfate solution is floated on top of the copper sulfate solution; The answer is that zinc is able to lose its outer electron more readily than copper. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition.
from en.wikipedia.org
Connecting the copper electrode to the zinc electrode. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The answer is that zinc is able to lose its outer electron more readily than copper. The positive sign of the copper e° shows that it releases electrons less readily. This phenomena occurs because copper metal is. [4] specific reactions include the reaction of the. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does.
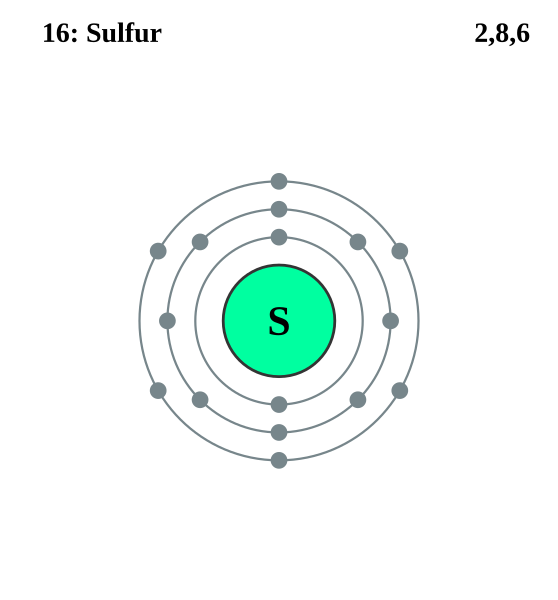
FileElectron shell 016 Sulfur.svg
Zinc Sulphate Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. [4] specific reactions include the reaction of the. A zinc sulfate solution is floated on top of the copper sulfate solution; The positive sign of the copper e° shows that it releases electrons less readily. Then a zinc electrode is placed in the zinc sulfate solution. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The answer is that zinc is able to lose its outer electron more readily than copper. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. Connecting the copper electrode to the zinc electrode. This phenomena occurs because copper metal is.
From schematicdatabitos99.z22.web.core.windows.net
Lewis Diagram Of Sulfur Zinc Sulphate Electrons [4] specific reactions include the reaction of the. The answer is that zinc is able to lose its outer electron more readily than copper. Then a zinc electrode is placed in the zinc sulfate solution. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and.. Zinc Sulphate Electrons.
From fphoto.photoshelter.com
science chemistry redox reaction zinc Fundamental Photographs The Zinc Sulphate Electrons This phenomena occurs because copper metal is. Then a zinc electrode is placed in the zinc sulfate solution. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Zinc Sulphate Electrons.
From sujatanutripharma.com
Zinc Sulphate Heptahydrate Sujata Nutri Pharma Zinc Sulphate Electrons A zinc sulfate solution is floated on top of the copper sulfate solution; This phenomena occurs because copper metal is. The positive sign of the copper e° shows that it releases electrons less readily. Then a zinc electrode is placed in the zinc sulfate solution. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. 93 rows you. Zinc Sulphate Electrons.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc Sulphate Electrons The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. A zinc sulfate solution is floated on top of the copper sulfate solution; Connecting the copper electrode to the zinc electrode. The positive sign of the copper e° shows that. Zinc Sulphate Electrons.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Zinc Sulphate Electrons A zinc sulfate solution is floated on top of the copper sulfate solution; It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. Connecting the copper electrode to the zinc electrode. [4] specific reactions include the reaction of the. Then. Zinc Sulphate Electrons.
From www.benjamin-mills.com
Electron configurations Zinc Sulphate Electrons The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. A zinc sulfate solution is floated on top of the copper sulfate solution; It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an. Zinc Sulphate Electrons.
From periodictable.me
Sulfur Electron Configuration (S) with Orbital Diagram Zinc Sulphate Electrons It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. [4] specific reactions include the reaction of the. The positive sign of the copper e° shows that it releases electrons less readily. Connecting the copper electrode to the zinc electrode. The answer is that zinc is able to lose its outer electron more readily than copper. The negative. Zinc Sulphate Electrons.
From smartlabid.com
Smart Lab Zinc Sulphate Electrons Then a zinc electrode is placed in the zinc sulfate solution. [4] specific reactions include the reaction of the. Connecting the copper electrode to the zinc electrode. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The negative sign of the zinc e° value shows. Zinc Sulphate Electrons.
From elchoroukhost.net
Periodic Table Sulfur Protons Neutrons Electrons Elcho Table Zinc Sulphate Electrons [4] specific reactions include the reaction of the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The positive sign of the copper e° shows that it releases electrons less readily. The answer is that zinc is able to lose its outer electron more readily. Zinc Sulphate Electrons.
From circuitwiringgear.z21.web.core.windows.net
Bohr Diagram Of Sulfur Zinc Sulphate Electrons A zinc sulfate solution is floated on top of the copper sulfate solution; The answer is that zinc is able to lose its outer electron more readily than copper. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. [4] specific reactions include the reaction of the. This phenomena occurs because copper. Zinc Sulphate Electrons.
From www.tradeindia.com
Zinc Sulphate Heptahydrate at Best Price in Rajkot, Gujarat Tanvi Bio Zinc Sulphate Electrons It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The answer is that zinc is able to lose its outer electron more readily than copper. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. What induces electrons to leave the atoms around. Zinc Sulphate Electrons.
From guweb2.gonzaga.edu
CHEM 101 Lecture 5 Zinc Sulphate Electrons What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. This phenomena occurs because copper metal is. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Zinc Sulphate Electrons.
From www.endeavourindustries.ca
Zinc Sulphate Hepta Hydrate 21 Endeavour Industries Canada Zinc Sulphate Electrons Then a zinc electrode is placed in the zinc sulfate solution. [4] specific reactions include the reaction of the. This phenomena occurs because copper metal is. A zinc sulfate solution is floated on top of the copper sulfate solution; Connecting the copper electrode to the zinc electrode. The negative sign of the zinc e° value shows that it releases electrons. Zinc Sulphate Electrons.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Sulphate Electrons What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. Connecting the copper electrode to the zinc electrode. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. [4] specific reactions include the reaction of the. Then a zinc electrode is placed in. Zinc Sulphate Electrons.
From www.indiamart.com
Zinc Sulphate Heptahydrate, 7733020 at Rs 33/kg in Ankleshwar ID Zinc Sulphate Electrons The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. Then a zinc electrode is placed in the zinc sulfate solution. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. The answer is that zinc is. Zinc Sulphate Electrons.
From epigenetics-international.com
Zinc Sulphate 200ml Zinc Sulphate Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. [4] specific reactions include the reaction of the. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. Connecting the copper electrode to the zinc electrode. Then a zinc electrode is placed in the. Zinc Sulphate Electrons.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Zinc Sulphate Electrons [4] specific reactions include the reaction of the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Connecting the copper electrode to the zinc electrode. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. What induces. Zinc Sulphate Electrons.
From www.newlandfert.com
Zinc Sulphate HeptahydrateNewland Resources Ltd Zinc Sulphate Electrons The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The answer is that zinc is able to lose its outer electron more readily than copper. Then a zinc electrode is placed in the zinc sulfate solution. [4] specific reactions. Zinc Sulphate Electrons.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Zinc Sulphate Electrons [4] specific reactions include the reaction of the. A zinc sulfate solution is floated on top of the copper sulfate solution; What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. The negative sign of the zinc e° value shows that it releases electrons more. Zinc Sulphate Electrons.
From ar.inspiredpencil.com
Electron Configuration Of Sulfur Zinc Sulphate Electrons The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. A zinc sulfate solution is floated on top of the copper sulfate solution; What induces electrons to leave. Zinc Sulphate Electrons.
From ar.inspiredpencil.com
Electron Configuration Of Sulfur Zinc Sulphate Electrons The positive sign of the copper e° shows that it releases electrons less readily. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. Connecting the copper electrode to the zinc electrode. The answer is that zinc is able to lose its outer electron more readily than copper. [4] specific reactions include the reaction of the. 93 rows. Zinc Sulphate Electrons.
From www.siyavula.com
4.6 Electronic configuration The atom Siyavula Zinc Sulphate Electrons [4] specific reactions include the reaction of the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. Then a zinc electrode is placed in the zinc sulfate. Zinc Sulphate Electrons.
From material-properties.org
Sulfur Protons Neutrons Electrons Electron Configuration Zinc Sulphate Electrons Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. What induces electrons to leave the atoms around the part of the zinc metal in a solution to. Zinc Sulphate Electrons.
From www.tradeindia.com
Zinc Sulphate Application Industrial at Best Price in Anand Waves Zinc Sulphate Electrons Then a zinc electrode is placed in the zinc sulfate solution. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. Connecting the copper electrode to the zinc electrode. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Zinc Sulphate Electrons.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc Sulphate Electrons The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. [4] specific reactions include the reaction of the. Then a zinc electrode is placed in the zinc sulfate solution. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc. Zinc Sulphate Electrons.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Sulphate Electrons The positive sign of the copper e° shows that it releases electrons less readily. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. [4] specific reactions include the reaction of the. This phenomena occurs because copper metal is. Then a zinc electrode is placed. Zinc Sulphate Electrons.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Zinc Sulphate Electrons Connecting the copper electrode to the zinc electrode. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. 93 rows you may assume the valences of the. Zinc Sulphate Electrons.
From www.indiamart.com
Zinc Sulphate Heptahydrate AR at Rs 180/kg Industrial Chemicals in Zinc Sulphate Electrons It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The positive sign of the copper e° shows that it releases electrons less readily. The answer is that zinc is able to lose its outer electron more readily than copper. Connecting the copper electrode to the zinc electrode. [4] specific reactions include the reaction of the. 93 rows. Zinc Sulphate Electrons.
From www.dreamstime.com
Atom of Sulfur with Core and 16 Electrons on White Stock Illustration Zinc Sulphate Electrons Then a zinc electrode is placed in the zinc sulfate solution. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. This phenomena occurs because copper metal is. What induces electrons to leave the atoms around the part of the. Zinc Sulphate Electrons.
From www.indiamart.com
Zinc Sulphate, For Agriculture at Rs 120/kg in Surat ID 2851322108912 Zinc Sulphate Electrons [4] specific reactions include the reaction of the. Then a zinc electrode is placed in the zinc sulfate solution. The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. A zinc sulfate solution is floated on top of the copper sulfate solution; 93 rows you may assume the valences of the chemical. Zinc Sulphate Electrons.
From stock.adobe.com
Sulfur atomic structure has atomic number, atomic mass, electron Zinc Sulphate Electrons Connecting the copper electrode to the zinc electrode. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. A zinc sulfate solution is floated on top of the copper sulfate solution; [4] specific. Zinc Sulphate Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Zinc Sulphate Electrons This phenomena occurs because copper metal is. [4] specific reactions include the reaction of the. The answer is that zinc is able to lose its outer electron more readily than copper. The positive sign of the copper e° shows that it releases electrons less readily. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The negative sign. Zinc Sulphate Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Sulfur (S, S2) Zinc Sulphate Electrons The positive sign of the copper e° shows that it releases electrons less readily. Connecting the copper electrode to the zinc electrode. This phenomena occurs because copper metal is. [4] specific reactions include the reaction of the. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc. Zinc Sulphate Electrons.
From en.wikipedia.org
FileElectron shell 016 Sulfur.svg Zinc Sulphate Electrons The negative sign of the zinc e° value shows that it releases electrons more readily than hydrogen does. It emits toxic fumes of zinc oxide and sulphur oxides during decomposition. The answer is that zinc is able to lose its outer electron more readily than copper. [4] specific reactions include the reaction of the. This phenomena occurs because copper metal. Zinc Sulphate Electrons.
From wirelistetiquette.z13.web.core.windows.net
Diagram Of Sulfur Zinc Sulphate Electrons What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A zinc sulfate solution is floated on top of the copper sulfate solution; 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Zinc Sulphate Electrons.