Boiling Point Of Pebbles . If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor.
from slideplayer.com
If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor.
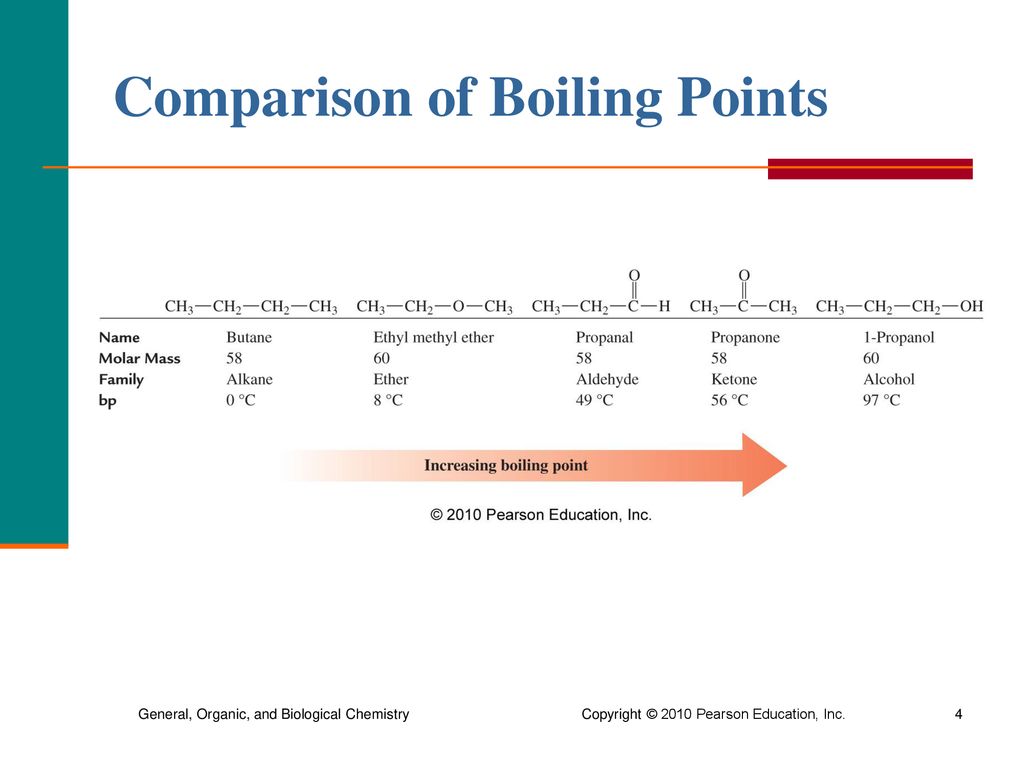
Chapter 14 Aldehydes, Ketones, and Chiral Molecules ppt download
Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor.
From slideplayer.com
Matter. ppt download Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding.. Boiling Point Of Pebbles.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru Boiling Point Of Pebbles boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. the melting points of. Boiling Point Of Pebbles.
From www.youtube.com
Boiling Points of Alkanes YouTube Boiling Point Of Pebbles 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. a compound's boiling point is a physical constant just like melting point, and so can be. Boiling Point Of Pebbles.
From slideplayer.com
which of these molecules has a permanent dipole (H2O, CO2, CCl4, NH3 Boiling Point Of Pebbles boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. a compound's boiling point. Boiling Point Of Pebbles.
From www.worksheetsplanet.com
What is Boiling Point Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. . Boiling Point Of Pebbles.
From www.chegg.com
Solved a) Create a Series containing the boiling points of Boiling Point Of Pebbles when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be. Boiling Point Of Pebbles.
From slideplayer.com
3.3.2 Alkanes. ppt download Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. boiling point, temperature at. Boiling Point Of Pebbles.
From www.slideserve.com
PPT 24.1 Petroleum Refining and the Hydrocarbons 24.2 Functional Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. the melting points of crystalline solids cannot be categorized in. Boiling Point Of Pebbles.
From slideplayer.com
Quiz What is an electrostatic dipole. ppt download Boiling Point Of Pebbles boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. the melting points of. Boiling Point Of Pebbles.
From slideplayer.com
Chapter 14 Aldehydes, Ketones, and Chiral Molecules ppt download Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. when we heat a liquid until it boils, the bubbles that form inside the liquid. Boiling Point Of Pebbles.
From www.mdpi.com
Energies Free FullText The Initial Boiling Point of Lubricating Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. . Boiling Point Of Pebbles.
From slidetodoc.com
THE ALKALI METALS Canvas lesson 04 The Alkali Boiling Point Of Pebbles the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. Boiling Point Of Pebbles.
From whistlecleanaustralia.com.au
Boiling Point Whistle Clean Australia Boiling Point Of Pebbles when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. a compound's boiling point is a physical constant just like melting point, and so can be used to support. Boiling Point Of Pebbles.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. . Boiling Point Of Pebbles.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Point Tessshebaylo Boiling Point Of Pebbles boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification. Boiling Point Of Pebbles.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Point Tessshebaylo Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of. Boiling Point Of Pebbles.
From loesrkqnt.blob.core.windows.net
Do Boiling Stones Lower Boiling Point at Raymond Spears blog Boiling Point Of Pebbles the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. when we heat a liquid until it boils, the bubbles that form inside the liquid consist of. Boiling Point Of Pebbles.
From www.pharmacalculation.com
Boiling Point Calculation Formula Boiling Point Of Pebbles when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. the melting points of crystalline solids cannot be categorized in as simple a fashion as. Boiling Point Of Pebbles.
From brainly.com
This graph shows the melting and boiling points of the alkali metals Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding.. Boiling Point Of Pebbles.
From www.slideserve.com
PPT STATES OF MATTER PowerPoint Presentation, free download ID2250162 Boiling Point Of Pebbles 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in. Boiling Point Of Pebbles.
From www.youtube.com
Boiling Point of Element Boiling. point of All metal What is boiling Boiling Point Of Pebbles the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. a compound's boiling point is a. Boiling Point Of Pebbles.
From askfilo.com
94. Calculate the boiling point of solution when 4 g of MgSO4 (M=120 g mo.. Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. Boiling Point Of Pebbles.
From pubchem.ncbi.nlm.nih.gov
Boiling Point Periodic Table of Elements PubChem Boiling Point Of Pebbles when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the. Boiling Point Of Pebbles.
From www.youtube.com
Calculate the boiling point of one molar aqueous solution (density 1.06 Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. the melting points of. Boiling Point Of Pebbles.
From slideplayer.com
Energy Changes & Phase Changes ppt download Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. . Boiling Point Of Pebbles.
From slideplayer.com
Phases of Matter Unit Notes ppt download Boiling Point Of Pebbles the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. If the. Boiling Point Of Pebbles.
From www.slideserve.com
PPT States, Boiling Point, Melting Point, and Solubility PowerPoint Boiling Point Of Pebbles when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. 100 rows the boiling. Boiling Point Of Pebbles.
From askfilo.com
The table given below shows the melting points and the boiling points of Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium. Boiling Point Of Pebbles.
From foodsafepal.com
Food Thermometers How to Calibrate and More FoodSafePal® Boiling Point Of Pebbles the melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled. Boiling Point Of Pebbles.
From www.pinterest.com
Boiling Point Boiling point, Distillation, Water boiling Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. . Boiling Point Of Pebbles.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. the melting points of. Boiling Point Of Pebbles.
From tipseri.com
What is the use of BRC in construction? Tipseri Boiling Point Of Pebbles If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. . Boiling Point Of Pebbles.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Of Pebbles a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature. . Boiling Point Of Pebbles.
From slideplayer.com
Do now Turn in Phases of Matter homework from Thursday. ppt download Boiling Point Of Pebbles 100 rows the boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. a compound's boiling point is a physical constant just like melting point, and so. Boiling Point Of Pebbles.
From facts.net
20 Fascinating Facts About Boiling Point Boiling Point Of Pebbles when we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. a compound's boiling point is a physical constant just like melting point, and so can be used to support the identification of a. 100 rows the boiling point of a substance is the temperature at which the vapor. Boiling Point Of Pebbles.