Titration Curve Formula . Titration curves for weak acid v strong base. A titration curve is a plot of some solution property versus the amount of added titrant. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Both equivalence points are visible. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. A titration curve is a graphical representation of the ph of a solution during a titration. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. The figure below shows two different. Initially, the ph of a weak acid like.
from www.chegg.com
Initially, the ph of a weak acid like. Suppose that a titration is performed and \(20.70 \: Titration curves for weak acid v strong base. The figure below shows two different. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a plot of some solution property versus the amount of added titrant. \ce{naoh}\) is required to reach the end point when titrated.
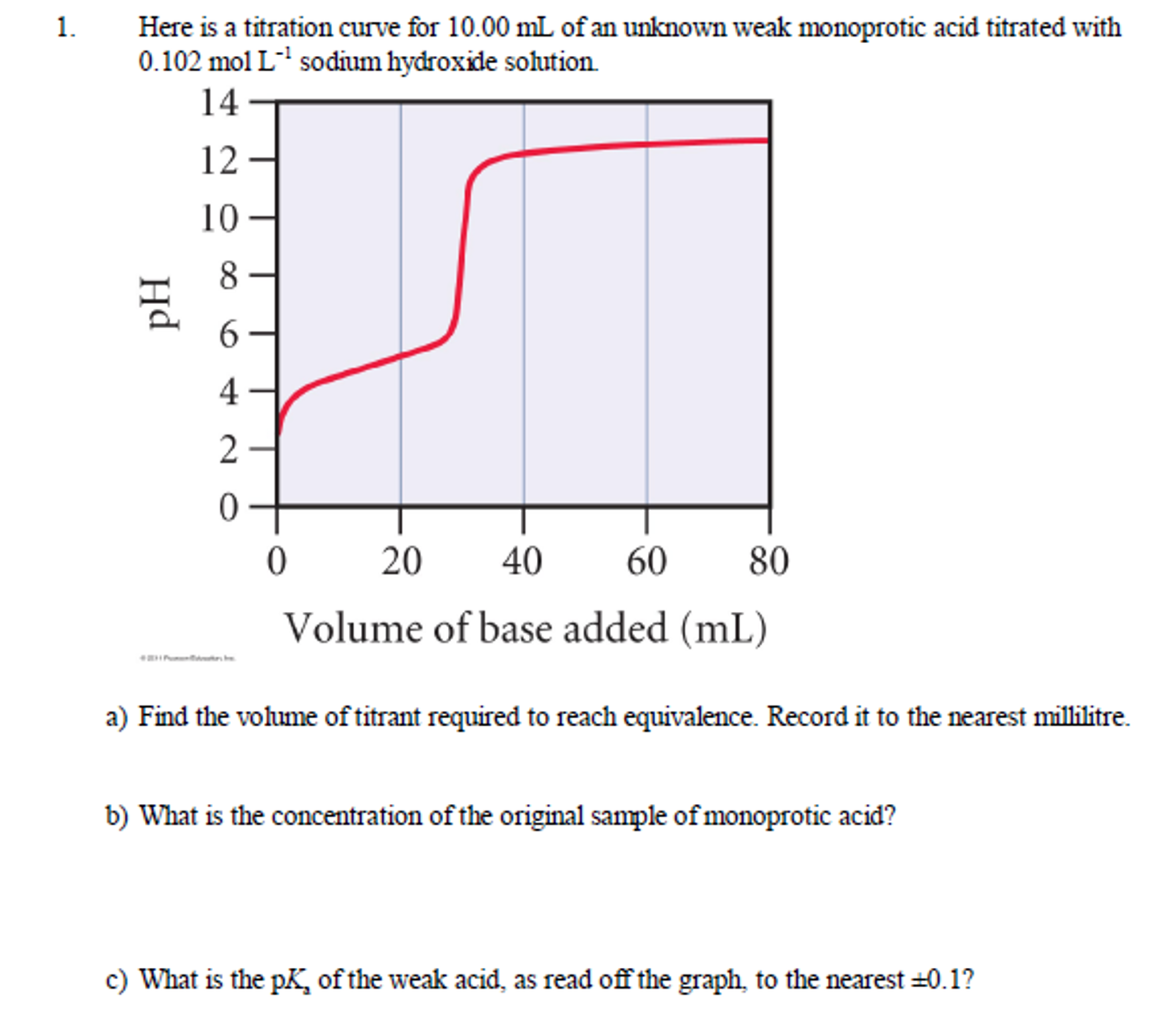
Solved Here is a titration curve for 10.00 mL of an unknown
Titration Curve Formula In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Both equivalence points are visible. Titration curves for weak acid v strong base. Suppose that a titration is performed and \(20.70 \: A titration curve is a graphical representation of the ph of a solution during a titration. \ce{naoh}\) is required to reach the end point when titrated. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. Initially, the ph of a weak acid like. A titration curve is a plot of some solution property versus the amount of added titrant. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Curve Formula A titration curve is a plot of some solution property versus the amount of added titrant. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. We'll take ethanoic acid and sodium hydroxide as typical of. Titration Curve Formula.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Formula Initially, the ph of a weak acid like. \ce{naoh}\) is required to reach the end point when titrated. Both equivalence points are visible. Titration curves for weak acid v strong base. The figure below shows two different. A titration curve is a plot of some solution property versus the amount of added titrant. Suppose that a titration is performed and. Titration Curve Formula.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Equilibrium Titration Curve Formula Titration curves for weak acid v strong base. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. Initially, the ph of a weak acid like. The figure below shows two different. A titration curve is a plot of some solution property versus the amount of added titrant. A typical titration curve of. Titration Curve Formula.
From www.pearson.com
The graphs labeled (a) and (b) show the titration curves for two Channels for Pearson+ Titration Curve Formula A titration curve is a plot of some solution property versus the amount of added titrant. Titration curves for weak acid v strong base. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the. Titration Curve Formula.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Formula A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Titration curves for weak acid v strong base. Both equivalence points are visible. \ce{naoh}\) is required to reach the end point when titrated. We'll take ethanoic. Titration Curve Formula.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Formula A titration curve is a plot of some solution property versus the amount of added titrant. Suppose that a titration is performed and \(20.70 \: In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a graphical representation of the ph of a solution during a titration. Initially, the. Titration Curve Formula.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Formula A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. \ce{naoh}\) is required to reach the end point when titrated. Initially, the ph of a weak acid like. In titrating a. Titration Curve Formula.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Formula A titration curve is a graphical representation of the ph of a solution during a titration. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. Titration curves for weak acid v strong base. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Suppose that. Titration Curve Formula.
From mungfali.com
Titration Curve Labeled Titration Curve Formula In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a graphical representation of the ph of a solution during a titration. Initially, the ph of a weak acid like. A titration curve is a plot of some solution property versus the amount of added titrant. We'll take ethanoic. Titration Curve Formula.
From www.researchgate.net
The calculated titration curves (equation 4 according to Table. 1) of... Download Scientific Titration Curve Formula Initially, the ph of a weak acid like. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Titration curves for weak acid v strong base. A titration curve is a graphical representation of. Titration Curve Formula.
From www.easybiologyclass.com
What is Titration Curve? What is pKa? EasyBiologyClass Titration Curve Formula \ce{naoh}\) is required to reach the end point when titrated. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a plot of some solution property versus the amount of added titrant. Both equivalence points are visible. We'll take ethanoic acid and sodium hydroxide as typical of a weak. Titration Curve Formula.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Formula A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. \ce{naoh}\) is required to reach the end point when titrated. Both equivalence points are visible. Titration curves for weak acid v strong base. Suppose that a. Titration Curve Formula.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Formula In titrating a weak acid with a strong base, different calculation methods are applied at various stages. \ce{naoh}\) is required to reach the end point when titrated. A titration curve is a plot of some solution property versus the amount of added titrant. Titration curves for weak acid v strong base. We'll take ethanoic acid and sodium hydroxide as typical. Titration Curve Formula.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Titration Curve Formula Both equivalence points are visible. Titration curves for weak acid v strong base. A titration curve is a plot of some solution property versus the amount of added titrant. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong. Titration Curve Formula.
From studylib.net
Titration Curve weak base with strong acid START Titration Curve Formula The figure below shows two different. \ce{naoh}\) is required to reach the end point when titrated. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Titration curves for weak acid v strong base. Suppose that a titration is performed and \(20.70 \: Both equivalence points are visible. A titration curve is a. Titration Curve Formula.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Formula A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of the ph of a solution during a titration. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. The figure below shows two different. A titration curve is. Titration Curve Formula.
From www.researchgate.net
Titration curves based on the exact equations (30), (14) and (26). C i... Download Scientific Titration Curve Formula Titration curves for weak acid v strong base. Initially, the ph of a weak acid like. A titration curve is a plot of some solution property versus the amount of added titrant. Both equivalence points are visible. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. \ce{naoh}\) is required to reach the. Titration Curve Formula.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Formula Titration curves for weak acid v strong base. A titration curve is a plot of some solution property versus the amount of added titrant. The figure below shows two different. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In titrating a weak acid with a strong base, different calculation methods are. Titration Curve Formula.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Formula A titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Suppose that a titration is performed and \(20.70 \: Both. Titration Curve Formula.
From mungfali.com
Endpoint Titration Curve Titration Curve Formula A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Suppose that a titration is performed and \(20.70 \: Both equivalence points are visible. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. \ce{naoh}\) is required to reach the end point when titrated. The figure. Titration Curve Formula.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Curve Formula Titration curves for weak acid v strong base. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. Initially, the ph of a weak acid like. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In titrating a weak acid with a strong base, different. Titration Curve Formula.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Formula Initially, the ph of a weak acid like. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. \ce{naoh}\) is required to reach the end point when titrated. The figure below shows two different. A titration curve is a graphical representation of the ph of a solution during a titration. In titrating a. Titration Curve Formula.
From chemwiki.ucdavis.edu
9B AcidBase Titrations Chemwiki Titration Curve Formula In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph of a weak acid like. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. A titration curve is a plot of some solution property versus the amount of added titrant.. Titration Curve Formula.
From www.tessshebaylo.com
Karl Fischer Titration Equation Tessshebaylo Titration Curve Formula We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. A titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Suppose that a titration is performed and \(20.70 \: In. Titration Curve Formula.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Formula A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a plot of some solution property versus the amount of added titrant. Both equivalence points are visible. \ce{naoh}\) is required to reach the end point when titrated. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid. Titration Curve Formula.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID4340170 Titration Curve Formula In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Both equivalence points are visible. The figure below shows two different. Initially, the ph of a weak acid like. Titration curves for weak acid v strong base. A titration curve is a graphical representation of the ph of a solution during a titration.. Titration Curve Formula.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Formula Both equivalence points are visible. A titration curve is a plot of some solution property versus the amount of added titrant. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Initially, the ph of a weak acid like. \ce{naoh}\) is required to reach the end point when titrated. A titration curve is. Titration Curve Formula.
From exonuxfur.blob.core.windows.net
Titration Curve Shape at Heather Jones blog Titration Curve Formula A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Suppose that a titration is performed and \(20.70 \: Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid. Titration Curve Formula.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Chemistry LibreTexts Titration Curve Formula In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Both equivalence points are visible. Initially, the ph of a weak acid like. Titration curves for weak acid v strong base. The figure below shows two. Titration Curve Formula.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve Formula A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph. Titration Curve Formula.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Formula The figure below shows two different. A titration curve is a plot of some solution property versus the amount of added titrant. Both equivalence points are visible. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In titrating a weak acid with a strong base, different calculation methods are applied at various. Titration Curve Formula.
From www.chegg.com
Solved Here is a titration curve for 10.00 mL of an unknown Titration Curve Formula A titration curve is a graphical representation of the ph of a solution during a titration. Suppose that a titration is performed and \(20.70 \: A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Titration curves for weak acid v strong base. The figure below shows two different. In titrating a weak. Titration Curve Formula.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Formula A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves for weak acid v strong base. \ce{naoh}\) is required to reach the end point when titrated. We'll take ethanoic acid and sodium hydroxide as typical of a weak acid and a strong base. The figure below shows two different. Initially, the ph. Titration Curve Formula.
From www.showme.com
Titration Curves Calculations Science, Chemicalreactions, Chemistry ShowMe Titration Curve Formula A titration curve is a plot of some solution property versus the amount of added titrant. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In titrating a weak acid with a strong base, different calculation methods are applied at various stages. A titration curve is a graphical representation of the ph. Titration Curve Formula.
From www.researchgate.net
Titration curves (experimental points, , and fits to the Kapp formula,... Download Scientific Titration Curve Formula Initially, the ph of a weak acid like. A titration curve is a plot of some solution property versus the amount of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. Suppose that a titration is performed and \(20.70 \: Titration curves for weak acid v strong base. In titrating a. Titration Curve Formula.