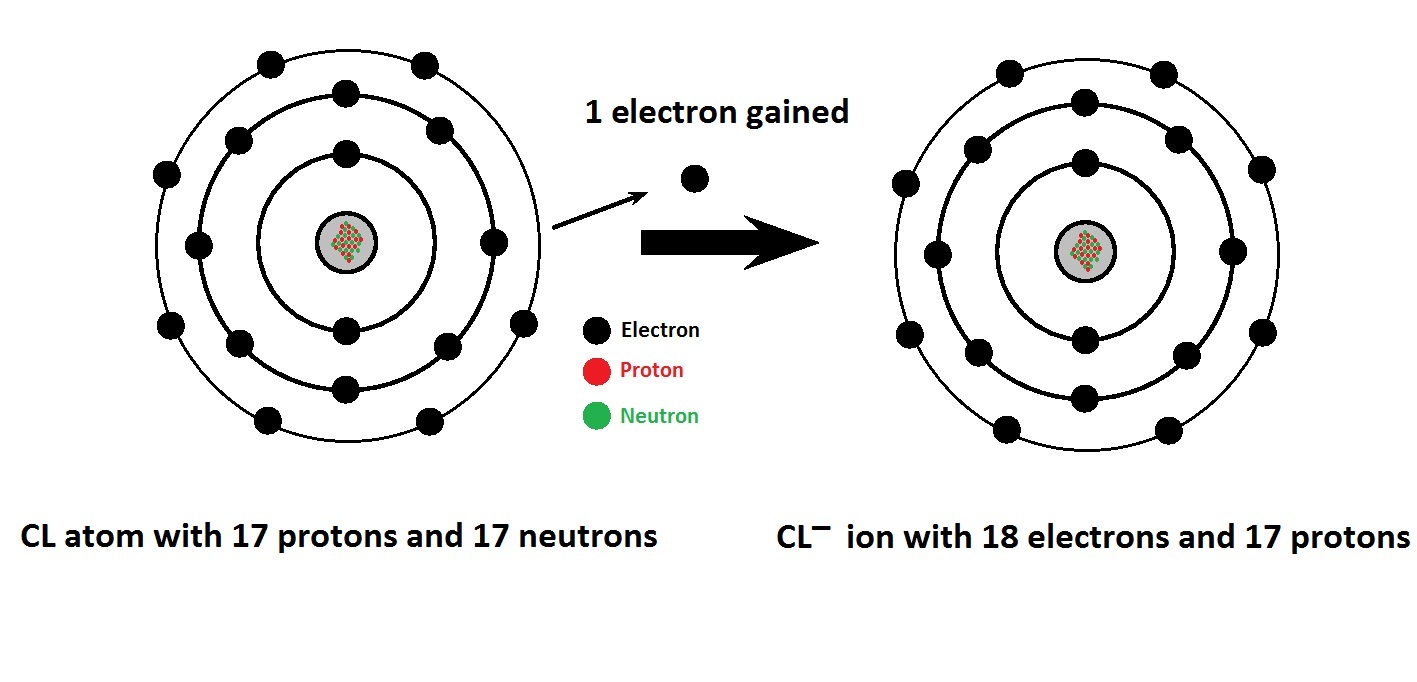
Chlorine Have A Charge . A chlorine atom starts with 17 electrons and 17 protons and is neutral. It is defined as being the charge that an atom would have if all bonds were ionic. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). This electric charge generated on the ion is. 93 rows ionic charge: 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Uncombined elements have an oxidation state of 0. On an industrial scale, chlorine is produced by. After gaining an electron to become an ion, it now. You can use this table to predict whether an atom can bond with another atom. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Using a simple, general trend for the ionic charge for elements on the periodic table, in this.
from basichemistry.blogspot.com
When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. You can use this table to predict whether an atom can bond with another atom. A chlorine atom starts with 17 electrons and 17 protons and is neutral. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. After gaining an electron to become an ion, it now. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). On an industrial scale, chlorine is produced by. 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). It is defined as being the charge that an atom would have if all bonds were ionic.
Basic Chemistry Ions, Cations, and Anions
Chlorine Have A Charge Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). After gaining an electron to become an ion, it now. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. 93 rows ionic charge: Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). This electric charge generated on the ion is. On an industrial scale, chlorine is produced by. A chlorine atom starts with 17 electrons and 17 protons and is neutral. It is defined as being the charge that an atom would have if all bonds were ionic. You can use this table to predict whether an atom can bond with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Have A Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. On an industrial scale, chlorine is produced by. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. After gaining an electron to become an ion, it now. It is defined as being the charge that an atom would. Chlorine Have A Charge.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Have A Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. After gaining an electron to become an ion, it now. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons,. Chlorine Have A Charge.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Have A Charge You can use this table to predict whether an atom can bond with another atom. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. This electric charge generated on the ion is. A chlorine atom starts with 17 electrons and 17 protons and is neutral. When these atoms gain electrons, they acquire. Chlorine Have A Charge.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Chlorine Have A Charge This electric charge generated on the ion is. A chlorine atom starts with 17 electrons and 17 protons and is neutral. On an industrial scale, chlorine is produced by. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). After gaining an electron to become an. Chlorine Have A Charge.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Chlorine Have A Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. A chlorine atom starts with 17 electrons and 17 protons and is neutral. It is defined as being the charge that. Chlorine Have A Charge.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Chlorine Have A Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. After gaining an electron to become an ion, it now. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). 93 rows this table. Chlorine Have A Charge.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Have A Charge After gaining an electron to become an ion, it now. It is defined as being the charge that an atom would have if all bonds were ionic. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Using a simple, general trend for the ionic charge for elements on the periodic table,. Chlorine Have A Charge.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Have A Charge 93 rows ionic charge: When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated on the ion is. Using a simple, general trend for the ionic charge for elements on the periodic table,. Chlorine Have A Charge.
From telgurus.co.uk
How to calculate number of neutrons, protons and electrons in Chlorine Chlorine Have A Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical elements. On an industrial scale, chlorine is produced by. You can use this table to predict. Chlorine Have A Charge.
From gardenandplate.com
Molecules Chlorine Have A Charge On an industrial scale, chlorine is produced by. You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the ion is. Uncombined elements have an oxidation state of 0. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Small amounts of. Chlorine Have A Charge.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. After gaining an electron to become an ion, it now. A chlorine atom starts with 17 electrons and 17 protons and is neutral. On. Chlorine Have A Charge.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows ionic charge: Using a simple, general trend for the ionic charge for elements on the periodic table, in this. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). After gaining an electron to become an. Chlorine Have A Charge.
From www.chegg.com
Solved Chlorine forms an ion with a charge of? a. 7− b. 2+ Chlorine Have A Charge On an industrial scale, chlorine is produced by. This electric charge generated on the ion is. Uncombined elements have an oxidation state of 0. You can use this table to predict whether an atom can bond with another atom. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an. Chlorine Have A Charge.
From slideplayer.com
Illustrating the bonding of Sodium and Chlorine ppt download Chlorine Have A Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a. Chlorine Have A Charge.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Have A Charge 93 rows ionic charge: Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). Using a simple, general trend for the ionic charge for elements on the periodic table, in this. You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges. Chlorine Have A Charge.
From ar.inspiredpencil.com
Chloride Ion Charge Chlorine Have A Charge When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. A chlorine atom starts with 17 electrons and 17 protons and is neutral. You can use this table to predict whether an atom can bond with another atom. Uncombined elements have an oxidation state of 0. On an industrial scale, chlorine is. Chlorine Have A Charge.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. This electric charge generated on the ion is. 93 rows ionic charge: Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). When these atoms gain electrons, they acquire a negative charge because they now possess more electrons. Chlorine Have A Charge.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Have A Charge Using a simple, general trend for the ionic charge for elements on the periodic table, in this. This electric charge generated on the ion is. It is defined as being the charge that an atom would have if all bonds were ionic. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.. Chlorine Have A Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Chlorine Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an oxidation state of 0. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.. Chlorine Have A Charge.
From www.numerade.com
SOLVED State the ionic charge for each of the following substances (a Chlorine Have A Charge Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). You can use this table to predict whether an atom can bond with another atom. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. When the atom loses or gains one or more electrons, the electric charge. Chlorine Have A Charge.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Have A Charge Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. This electric charge generated on the ion is. On an industrial scale, chlorine is produced by. Using a simple, general trend for the ionic charge for. Chlorine Have A Charge.
From www.britannica.com
Chlorine Uses, Properties, & Facts Britannica Chlorine Have A Charge After gaining an electron to become an ion, it now. On an industrial scale, chlorine is produced by. You can use this table to predict whether an atom can bond with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Uncombined elements have an oxidation state of. Chlorine Have A Charge.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chlorine Have A Charge On an industrial scale, chlorine is produced by. A chlorine atom starts with 17 electrons and 17 protons and is neutral. After gaining an electron to become an ion, it now. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges. Chlorine Have A Charge.
From sciencenotes.org
Chlorine Facts Chlorine Have A Charge Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). Uncombined elements have an oxidation state of 0. You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. When these atoms gain electrons, they acquire. Chlorine Have A Charge.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Have A Charge On an industrial scale, chlorine is produced by. Uncombined elements have an oxidation state of 0. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. 93 rows ionic charge: 93 rows this table shows the most. Chlorine Have A Charge.
From ar.inspiredpencil.com
Chloride Ion Charge Chlorine Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). 93 rows this table. Chlorine Have A Charge.
From telgurus.co.uk
How to calculate number of neutrons, protons and electrons in Chlorine Chlorine Have A Charge After gaining an electron to become an ion, it now. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical elements. On an industrial scale, chlorine is produced by. When the atom loses or gains one or more electrons,. Chlorine Have A Charge.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4192871 Chlorine Have A Charge 93 rows ionic charge: You can use this table to predict whether an atom can bond with another atom. After gaining an electron to become an ion, it now. This electric charge generated on the ion is. On an industrial scale, chlorine is produced by. Using a simple, general trend for the ionic charge for elements on the periodic table,. Chlorine Have A Charge.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Have A Charge On an industrial scale, chlorine is produced by. This electric charge generated on the ion is. It is defined as being the charge that an atom would have if all bonds were ionic. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Uncombined elements have an oxidation state of 0. 93. Chlorine Have A Charge.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).. Chlorine Have A Charge.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Have A Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. 93 rows this table shows the most common charges for atoms of the chemical elements. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). Uncombined elements have an oxidation state of 0. After gaining an electron to become an ion, it. Chlorine Have A Charge.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Have A Charge You can use this table to predict whether an atom can bond with another atom. After gaining an electron to become an ion, it now. 93 rows ionic charge: 93 rows this table shows the most common charges for atoms of the chemical elements. A chlorine atom starts with 17 electrons and 17 protons and is neutral. This electric charge. Chlorine Have A Charge.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Have A Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. On an industrial scale, chlorine is produced by. Uncombined elements have an oxidation state of 0. You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. When. Chlorine Have A Charge.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Have A Charge After gaining an electron to become an ion, it now. Using a simple, general trend for the ionic charge for elements on the periodic table, in this. A chlorine atom starts with 17 electrons and 17 protons and is neutral. Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of. Chlorine Have A Charge.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains Chlorine Have A Charge A chlorine atom starts with 17 electrons and 17 protons and is neutral. Small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). 93 rows ionic charge: It is defined as being the charge that an atom would have if all bonds were ionic. When these atoms gain electrons, they acquire a negative charge because. Chlorine Have A Charge.