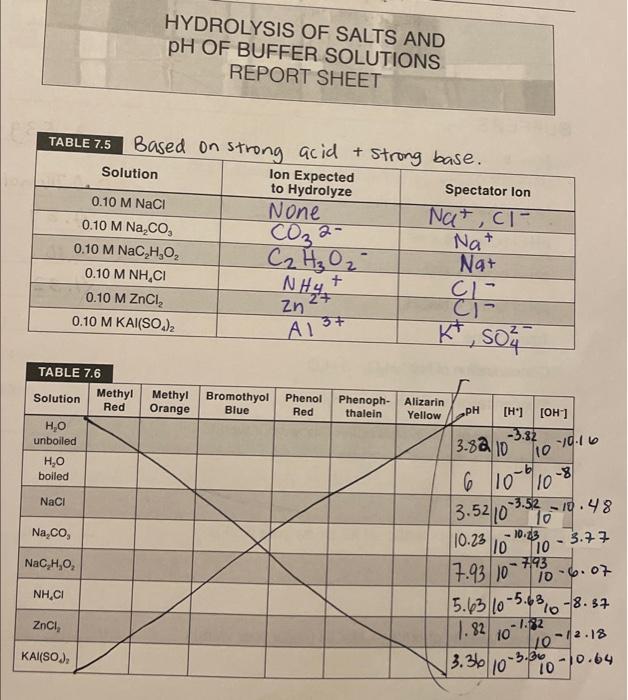
Hydrolysis Of Salts Lab Report . To gain familiarization with the concept of hydrolysis. The approximate ph of these. Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Calculate the concentrations of the various species in a salt solution; It is different from simple dissolution. Hydrolysis of salts and buffer system calculations objective: Introduction hydrolysis is a chemical reaction of a compound with water. When an ionic compound (salt). Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. Predict whether a salt solution will be acidic, basic, or neutral; Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water?
from www.chegg.com
Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. Calculate the concentrations of the various species in a salt solution; Introduction hydrolysis is a chemical reaction of a compound with water. Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Predict whether a salt solution will be acidic, basic, or neutral; To gain familiarization with the concept of hydrolysis. Hydrolysis of salts and buffer system calculations objective: Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? When an ionic compound (salt). It is different from simple dissolution.
Solved LABORATORY 7 HYDROLYSIS OF SALTS AND PH OF BUFFER
Hydrolysis Of Salts Lab Report Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. To gain familiarization with the concept of hydrolysis. Hydrolysis of salts and buffer system calculations objective: It is different from simple dissolution. The approximate ph of these. Calculate the concentrations of the various species in a salt solution; Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. When an ionic compound (salt). Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Predict whether a salt solution will be acidic, basic, or neutral; Introduction hydrolysis is a chemical reaction of a compound with water.
From myans.bhantedhammika.net
Hydrolysis Of Salts Lab Answers Hydrolysis Of Salts Lab Report It is different from simple dissolution. Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. Hydrolysis of salts and buffer system calculations objective: When an ionic compound (salt). Predict whether a salt solution will be acidic, basic, or neutral; To gain familiarization with. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved LABORATORY 7 HYDROLYSIS OF SALTS AND PH OF BUFFER Hydrolysis Of Salts Lab Report Introduction hydrolysis is a chemical reaction of a compound with water. Calculate the concentrations of the various species in a salt solution; Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? When an ionic compound (salt). To gain familiarization with the concept of hydrolysis. Post 6 hydrolysis. Hydrolysis Of Salts Lab Report.
From www.youtube.com
Chemistry Acids and Bases Hydrolysis of salts (Simplified) YouTube Hydrolysis Of Salts Lab Report Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Introduction hydrolysis is a chemical reaction of a compound with water. To gain familiarization. Hydrolysis Of Salts Lab Report.
From www.researchgate.net
Salt hydrolysis process. Download Scientific Diagram Hydrolysis Of Salts Lab Report Hydrolysis of salts and buffer system calculations objective: It is different from simple dissolution. Introduction hydrolysis is a chemical reaction of a compound with water. Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Post 6 hydrolysis of salts and ph of buffer solutions introduction when a. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved HYDROLYSIS OF SALTS AND pH OF BUFFER SOLUTIONS REPORT Hydrolysis Of Salts Lab Report It is different from simple dissolution. Hydrolysis of salts and buffer system calculations objective: When an ionic compound (salt). To gain familiarization with the concept of hydrolysis. Calculate the concentrations of the various species in a salt solution; Introduction hydrolysis is a chemical reaction of a compound with water. The approximate ph of these. Salt hydrolysis is a reaction in. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved 2 328 Report Sheet Hydrolysis of Salts and pH of Hydrolysis Of Salts Lab Report Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. Calculate the concentrations of the various species in a salt solution; To. Hydrolysis Of Salts Lab Report.
From classnotes.ng
Hydrolysis of Salt ClassNotes.ng Hydrolysis Of Salts Lab Report Calculate the concentrations of the various species in a salt solution; Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. It is different from simple dissolution. The approximate ph of these. Which of the following reactions best represents the hydrolysis reaction of a. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved у Hydrolysis of Salts Calculations Table 3d Hydrolysis Of Salts Lab Report Hydrolysis of salts and buffer system calculations objective: When an ionic compound (salt). It is different from simple dissolution. Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. Salt hydrolysis is a reaction in which one of the ions from a salt reacts. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved Report 8A Soluble Salts and the Hydrolysis Concept Hydrolysis Of Salts Lab Report Predict whether a salt solution will be acidic, basic, or neutral; When an ionic compound (salt). The approximate ph of these. To gain familiarization with the concept of hydrolysis. Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Hydrolysis of salts and buffer system calculations. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Report 8A Soluble Salts and the Hydrolysis Concept Hydrolysis Of Salts Lab Report The approximate ph of these. Calculate the concentrations of the various species in a salt solution; It is different from simple dissolution. Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved 328 Report Sheet Hydrolysis of salts and pH of Buffer Hydrolysis Of Salts Lab Report Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. When an ionic compound (salt). Introduction hydrolysis is a chemical reaction of a compound with water. It is different from simple dissolution. Which of the following reactions best represents the hydrolysis reaction of a salt that. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved REPORT SHEET Hydrolysis of Salts and pH of Buffer Hydrolysis Of Salts Lab Report When an ionic compound (salt). Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. It is different from simple dissolution. Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Introduction hydrolysis is. Hydrolysis Of Salts Lab Report.
From myans.bhantedhammika.net
Hydrolysis Of Salts Lab Answers Hydrolysis Of Salts Lab Report To gain familiarization with the concept of hydrolysis. Introduction hydrolysis is a chemical reaction of a compound with water. Hydrolysis of salts and buffer system calculations objective: Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. It is different from simple dissolution. Which. Hydrolysis Of Salts Lab Report.
From myans.bhantedhammika.net
Hydrolysis Of Salts Lab Answers Hydrolysis Of Salts Lab Report Calculate the concentrations of the various species in a salt solution; It is different from simple dissolution. When an ionic compound (salt). Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or. Hydrolysis Of Salts Lab Report.
From www.studocu.com
Lab 7 Chem 162 Chem 162 Lab Manual for Lab 7 HYDROLYSIS OF SALTS Hydrolysis Of Salts Lab Report Calculate the concentrations of the various species in a salt solution; Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? When an ionic compound (salt). It is different from simple dissolution. Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Hydrolysis of Salts 24 and pH Hydrolysis Of Salts Lab Report The approximate ph of these. Calculate the concentrations of the various species in a salt solution; Predict whether a salt solution will be acidic, basic, or neutral; When an ionic compound (salt). To gain familiarization with the concept of hydrolysis. It is different from simple dissolution. Hydrolysis of salts and buffer system calculations objective: Salt hydrolysis is a reaction in. Hydrolysis Of Salts Lab Report.
From www.scribd.com
Hydrolysis of Salt & PH of Buffer Solution (Sample) PDF Acid Salt Hydrolysis Of Salts Lab Report Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? To gain familiarization with the concept of hydrolysis. Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. Predict whether a salt. Hydrolysis Of Salts Lab Report.
From printablezonebunias.z21.web.core.windows.net
Hydrolysis Of Salts Lab Answers Hydrolysis Of Salts Lab Report The approximate ph of these. When an ionic compound (salt). Hydrolysis of salts and buffer system calculations objective: Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved LABORATORY 7 HYDROLYSIS OF SALTS AND pH OF BUFFER Hydrolysis Of Salts Lab Report Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Which of the following reactions best represents the hydrolysis reaction. Hydrolysis Of Salts Lab Report.
From www.youtube.com
What is salt and salt hydrolysis YouTube Hydrolysis Of Salts Lab Report When an ionic compound (salt). Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. To gain familiarization with the concept of hydrolysis. It. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved REPORT SHEET Hydrolysis of Salts and pH of Buffer Hydrolysis Of Salts Lab Report Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? To gain familiarization with the concept of hydrolysis. Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. The approximate ph of these. It. Hydrolysis Of Salts Lab Report.
From myans.bhantedhammika.net
Hydrolysis Of Salts And Ph Of Buffer Solutions Hydrolysis Of Salts Lab Report Calculate the concentrations of the various species in a salt solution; It is different from simple dissolution. Hydrolysis of salts and buffer system calculations objective: Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. The approximate ph of these. Which of the following reactions best. Hydrolysis Of Salts Lab Report.
From myans.bhantedhammika.net
Hydrolysis Of Salts And Ph Of Buffer Solutions Hydrolysis Of Salts Lab Report When an ionic compound (salt). Introduction hydrolysis is a chemical reaction of a compound with water. The approximate ph of these. Hydrolysis of salts and buffer system calculations objective: Predict whether a salt solution will be acidic, basic, or neutral; To gain familiarization with the concept of hydrolysis. Salt hydrolysis is a reaction in which one of the ions from. Hydrolysis Of Salts Lab Report.
From myans.bhantedhammika.net
Hydrolysis Of Salts And Ph Of Buffer Solutions Hydrolysis Of Salts Lab Report The approximate ph of these. Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Introduction hydrolysis is a chemical reaction of a compound. Hydrolysis Of Salts Lab Report.
From www.chegg.com
LABORATORY 7E HYDROLYSIS OF SALTS AND pH OF BUFFER Hydrolysis Of Salts Lab Report Hydrolysis of salts and buffer system calculations objective: Introduction hydrolysis is a chemical reaction of a compound with water. To gain familiarization with the concept of hydrolysis. Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. Predict whether a salt solution will be acidic, basic,. Hydrolysis Of Salts Lab Report.
From myans.bhantedhammika.net
Hydrolysis Of Salt Pdf Hydrolysis Of Salts Lab Report When an ionic compound (salt). Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. The approximate ph of these. Calculate the. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMI Hydrolysis Of Salts 24 And P... Hydrolysis Of Salts Lab Report Calculate the concentrations of the various species in a salt solution; It is different from simple dissolution. Predict whether a salt solution will be acidic, basic, or neutral; Hydrolysis of salts and buffer system calculations objective: Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? To gain. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved 328 Report Sheet . Hydrolysis of Salts and pH of Hydrolysis Of Salts Lab Report It is different from simple dissolution. Hydrolysis of salts and buffer system calculations objective: To gain familiarization with the concept of hydrolysis. When an ionic compound (salt). Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Post 6 hydrolysis of salts and ph of buffer solutions introduction. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Hydrolysis of Salts 24 and pH Hydrolysis Of Salts Lab Report Calculate the concentrations of the various species in a salt solution; Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. When an ionic compound (salt). It is different from simple dissolution. Introduction hydrolysis is a chemical reaction of a compound with water. To. Hydrolysis Of Salts Lab Report.
From www.researchgate.net
(PDF) Hydrolysis of Salts Hydrolysis Of Salts Lab Report When an ionic compound (salt). Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? The approximate ph of these. Calculate the concentrations of the various species in a salt solution; To gain familiarization with the concept of hydrolysis. Introduction hydrolysis is a chemical reaction of a compound. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved LABORATORY 71 HYDROLYSIS OF SALTS AND pH OF BUFFER Hydrolysis Of Salts Lab Report Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? When an ionic compound (salt). Predict whether a salt solution will be acidic, basic, or neutral; Calculate the concentrations of the various species in a salt solution; It is different from simple dissolution. Salt hydrolysis is a reaction. Hydrolysis Of Salts Lab Report.
From www.studocu.com
AcidBase Properties of Salt Solutions Hydrolysis lab This is known Hydrolysis Of Salts Lab Report When an ionic compound (salt). Introduction hydrolysis is a chemical reaction of a compound with water. It is different from simple dissolution. Post 6 hydrolysis of salts and ph of buffer solutions introduction when a weak acid or weak basic salt is dissolved in water or both, a. The approximate ph of these. Predict whether a salt solution will be. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved Report Sheet Hydrolysis of salt Solution pH [H] Hydrolysis Of Salts Lab Report Predict whether a salt solution will be acidic, basic, or neutral; Introduction hydrolysis is a chemical reaction of a compound with water. The approximate ph of these. Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. To gain familiarization with the concept of hydrolysis. Which. Hydrolysis Of Salts Lab Report.
From myans.bhantedhammika.net
Hydrolysis Of Salts And Ph Of Buffer Solutions Hydrolysis Of Salts Lab Report Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. To gain familiarization with the concept of hydrolysis. Which of the following reactions best represents the hydrolysis reaction of a salt that gives an acidic solution when dissolved in water? Calculate the concentrations of the various. Hydrolysis Of Salts Lab Report.
From www.chegg.com
Solved Experiment 6 Hydrolysis of Salts Report/Data Sheet Hydrolysis Of Salts Lab Report Salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic solution. When an ionic compound (salt). Predict whether a salt solution will be acidic, basic, or neutral; Introduction hydrolysis is a chemical reaction of a compound with water. Hydrolysis of salts and buffer system calculations objective: The. Hydrolysis Of Salts Lab Report.