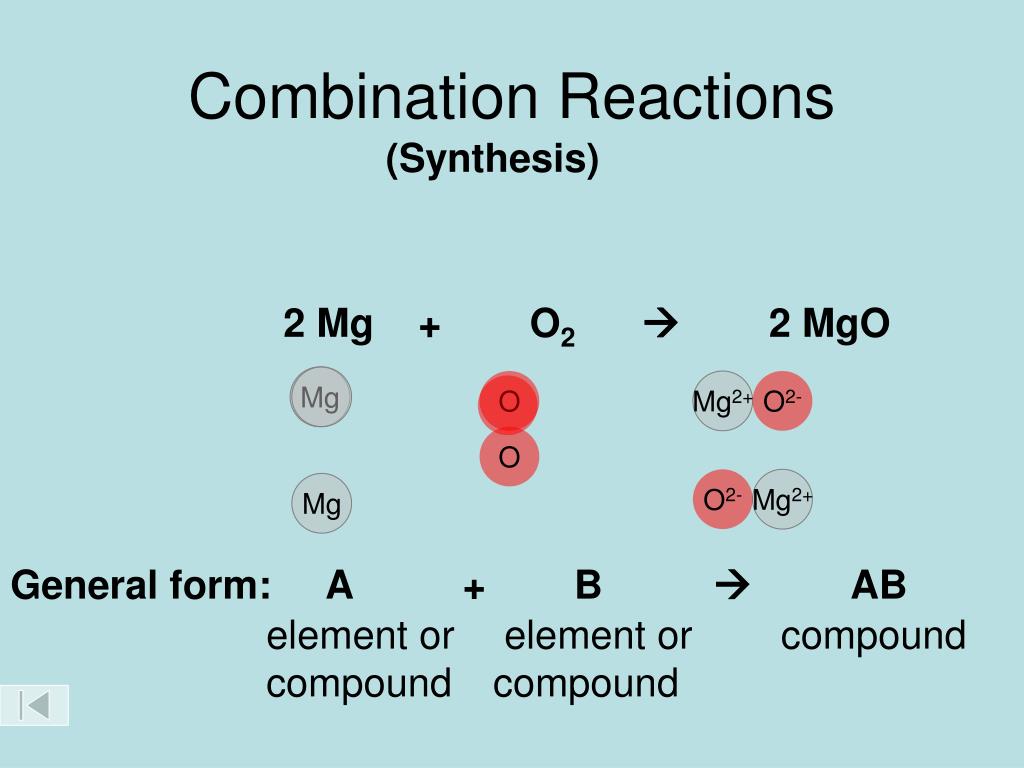
List Of Combination Reactions . 2h2 + o2 → 2h2o. A combination reaction is a reaction in which two or more substances combine to form a single new substance. 10 examples of combination reactions. Combination reaction may occur between two elements, two compounds, or between a compound and an element. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. C + o 2 → co 2. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). Combination reactions describe a reaction like this: In this reaction, two reactants become a single product. The reaction between iron and sulfur to form. Combination reactions can also be. The reaction between hydrogen gas and oxygen gas to form water: The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: The reactants may be elements or compounds, while the product is always a compound. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of.
from www.vrogue.co
10 examples of combination reactions. Combination reaction may occur between two elements, two compounds, or between a compound and an element. The reaction between iron and sulfur to form. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). The reaction between hydrogen gas and oxygen gas to form water: C + o 2 → co 2. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: Combination reactions describe a reaction like this: Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. In this reaction, two reactants become a single product.
Types Of Chemical Reaction Combination Reaction Ncert vrogue.co
List Of Combination Reactions 2h2 + o2 → 2h2o. 2h2 + o2 → 2h2o. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Combination reactions describe a reaction like this: The reaction between iron and sulfur to form. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. Combination reaction may occur between two elements, two compounds, or between a compound and an element. In this reaction, two reactants become a single product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: C + o 2 → co 2. The reactants may be elements or compounds, while the product is always a compound. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). Combination reactions can also be. The reaction between hydrogen gas and oxygen gas to form water: 10 examples of combination reactions.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID List Of Combination Reactions 2h2 + o2 → 2h2o. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. The reaction between hydrogen gas and oxygen gas to form water: A combination reaction is a reaction in which two or more substances combine to form a single new substance. The reactants may. List Of Combination Reactions.
From www.sliderbase.com
Reaction Types Presentation Chemistry List Of Combination Reactions Combination reaction may occur between two elements, two compounds, or between a compound and an element. In this reaction, two reactants become a single product. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). 2h2 + o2 → 2h2o. Though chemical reactions can be as complex as they are numerous, they are all. List Of Combination Reactions.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) List Of Combination Reactions In this reaction, two reactants become a single product. The reaction between hydrogen gas and oxygen gas to form water: Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. Combination reactions can also be. The burning of carbon in the presence of oxygen to produce carbon dioxide. List Of Combination Reactions.
From www.slideserve.com
PPT Classifying Chemical Reactions PowerPoint Presentation, free List Of Combination Reactions The reactants may be elements or compounds, while the product is always a compound. The reaction between iron and sulfur to form. In this reaction, two reactants become a single product. 10 examples of combination reactions. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: A combination reaction is a reaction in. List Of Combination Reactions.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID745735 List Of Combination Reactions C + o 2 → co 2. Combination reaction may occur between two elements, two compounds, or between a compound and an element. The reactants may be elements or compounds, while the product is always a compound. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also. List Of Combination Reactions.
From www.slideserve.com
PPT CHEMICAL REACTIONS PowerPoint Presentation, free download ID List Of Combination Reactions A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Combination reactions describe a reaction like this: Combination reactions can also be. 2h2 + o2 → 2h2o. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination. List Of Combination Reactions.
From www.chemistrylearner.com
Chemical Reactions Types, Definitions, and Examples List Of Combination Reactions Combination reactions can also be. In this reaction, two reactants become a single product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reaction may occur between two elements, two compounds, or between a compound and an element. Though chemical reactions can be as complex as they are numerous,. List Of Combination Reactions.
From mavink.com
Combination Chemical Reaction Examples List Of Combination Reactions Combination reactions describe a reaction like this: \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). Combination reactions can also be. 10 examples of combination reactions. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: A combination reaction is a reaction in which two or. List Of Combination Reactions.
From www.slideserve.com
PPT Ionic Chemical Reactions PowerPoint Presentation, free download List Of Combination Reactions 2h2 + o2 → 2h2o. C + o 2 → co 2. The reactants may be elements or compounds, while the product is always a compound. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). The reaction between iron and sulfur to form. Combination reactions describe a reaction like this: 10 examples of. List Of Combination Reactions.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free List Of Combination Reactions The reaction between iron and sulfur to form. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: The reactants may be elements or compounds, while the product is always a compound. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). In this reaction, two reactants. List Of Combination Reactions.
From geteducationskills.com
Latest Chapter on Combination Reaction With Example Get Education List Of Combination Reactions The reaction between hydrogen gas and oxygen gas to form water: Combination reaction may occur between two elements, two compounds, or between a compound and an element. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). 2h2 + o2 → 2h2o. A combination reaction is a reaction in which two or more substances. List Of Combination Reactions.
From www.slideserve.com
PPT Types of Reactions & Combination Reactions (7.57.6) PowerPoint List Of Combination Reactions The reaction between hydrogen gas and oxygen gas to form water: In this reaction, two reactants become a single product. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. The reactants may be elements or compounds, while the product is always a compound. C + o 2. List Of Combination Reactions.
From slideplayer.com
Chemical Reactions *5 General Types Combination ppt List Of Combination Reactions Combination reaction may occur between two elements, two compounds, or between a compound and an element. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to. List Of Combination Reactions.
From study.com
How to Identify a Combination Reaction Chemistry List Of Combination Reactions The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. The reaction between hydrogen gas and oxygen gas to form water: Combination reactions can also be. The reactants may be elements. List Of Combination Reactions.
From www.teachoo.com
NCERT Exemplar (MCQ) Which of the following are combination reaction List Of Combination Reactions \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). The reaction between iron and sulfur to form. C + o 2 → co 2. 2h2 + o2 → 2h2o. Combination reaction may occur between two elements, two compounds, or between a compound and an element. 10 examples of combination reactions. The burning of. List Of Combination Reactions.
From colouremployer8.gitlab.io
Supreme 4 Types Of Chemical Reactions Reactants And Products Cellular List Of Combination Reactions Combination reactions describe a reaction like this: In this reaction, two reactants become a single product. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reaction may occur between two elements, two compounds, or between a compound and an element. The reaction between iron and sulfur to form. The. List Of Combination Reactions.
From www.slideshare.net
Types of reactions year 10 List Of Combination Reactions Combination reactions describe a reaction like this: 10 examples of combination reactions. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. A combination reaction is a reaction in which two or more substances combine to form a single new substance. A synthesis reaction or direct combination reaction. List Of Combination Reactions.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID List Of Combination Reactions In this reaction, two reactants become a single product. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. Combination reactions describe a reaction like this: Combination reaction may occur between two elements, two compounds, or between a compound and an element. A synthesis reaction or direct combination. List Of Combination Reactions.
From www.slideserve.com
PPT Types of Reactions and Combination Reactions PowerPoint List Of Combination Reactions Combination reaction may occur between two elements, two compounds, or between a compound and an element. The reactants may be elements or compounds, while the product is always a compound. The reaction between hydrogen gas and oxygen gas to form water: Combination reactions can also be. A synthesis reaction or direct combination reaction is a type of chemical reaction in. List Of Combination Reactions.
From www.slideserve.com
PPT A Study of Chemical Reactions PowerPoint Presentation, free List Of Combination Reactions Combination reactions describe a reaction like this: Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A combination reaction is. List Of Combination Reactions.
From www.vrogue.co
Types Of Chemical Reaction Combination Reaction Ncert vrogue.co List Of Combination Reactions A combination reaction is a reaction in which two or more substances combine to form a single new substance. The reaction between iron and sulfur to form. 2h2 + o2 → 2h2o. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). The reaction between hydrogen gas and oxygen gas to form water: A. List Of Combination Reactions.
From www.goodscience.com.au
Types of Chemical Reactions Good Science List Of Combination Reactions Combination reactions describe a reaction like this: The reaction between hydrogen gas and oxygen gas to form water: In this reaction, two reactants become a single product. Combination reactions can also be. C + o 2 → co 2. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws. List Of Combination Reactions.
From www.youtube.com
Types of chemical reactions reaction, Exothermic List Of Combination Reactions The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. 2h2 + o2 → 2h2o. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two. List Of Combination Reactions.
From byjus.com
Types of Chemical Reactions Detailed Explanation With Example & Videos List Of Combination Reactions A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. C + o 2 → co 2. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reaction may occur between two elements,. List Of Combination Reactions.
From www.slideserve.com
PPT Chapter 8 Chemical Reactions PowerPoint Presentation, free List Of Combination Reactions A combination reaction is a reaction in which two or more substances combine to form a single new substance. A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. Combination reactions describe a reaction like this: C + o 2 → co 2.. List Of Combination Reactions.
From slideplayer.com
TYPES OF CHEMICAL REACTIONS ppt download List Of Combination Reactions The reactants may be elements or compounds, while the product is always a compound. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be. \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). C + o 2 → co. List Of Combination Reactions.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free List Of Combination Reactions The reaction between hydrogen gas and oxygen gas to form water: The reactants may be elements or compounds, while the product is always a compound. Though chemical reactions can be as complex as they are numerous, they are all fundamentally governed by these same guiding laws of. 2h2 + o2 → 2h2o. In this reaction, two reactants become a single. List Of Combination Reactions.
From studyqueries.com
What Is Combination Reaction? Definition, Examples List Of Combination Reactions The reaction between hydrogen gas and oxygen gas to form water: The reaction between iron and sulfur to form. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a. List Of Combination Reactions.
From www.slideserve.com
PPT Types of Reactions & Combination Reactions (7.57.6) PowerPoint List Of Combination Reactions 10 examples of combination reactions. The reaction between hydrogen gas and oxygen gas to form water: Combination reactions describe a reaction like this: 2h2 + o2 → 2h2o. Combination reaction may occur between two elements, two compounds, or between a compound and an element. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination. List Of Combination Reactions.
From www.yaclass.in
Combination reaction Examples — lesson. Science CBSE, Class 10. List Of Combination Reactions A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reaction between iron and sulfur to form. C + o 2 → co 2. A combination reaction is a reaction in which two or more substances combine to form a single new. List Of Combination Reactions.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free List Of Combination Reactions The reaction between iron and sulfur to form. 2h2 + o2 → 2h2o. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reaction may occur between two elements, two compounds, or between a compound and an element. A synthesis reaction or direct combination reaction is a type of chemical. List Of Combination Reactions.
From www.teachoo.com
Combination Reaction Definition with 4 Examples Class 10 Chemistry List Of Combination Reactions The reaction between hydrogen gas and oxygen gas to form water: 10 examples of combination reactions. In this reaction, two reactants become a single product. The reactants may be elements or compounds, while the product is always a compound. Combination reaction may occur between two elements, two compounds, or between a compound and an element. The burning of carbon in. List Of Combination Reactions.
From slidetodoc.com
Chemical Equations Reactions Chemistry A Chemical Reactions You List Of Combination Reactions \[a + b \rightarrow c\] in which two or more reactants become one product (are combined). Combination reaction may occur between two elements, two compounds, or between a compound and an element. The reactants may be elements or compounds, while the product is always a compound. A combination reaction is a reaction in which two or more substances combine to. List Of Combination Reactions.
From www.pinterest.fr
This diagram gives a summary of the four types of reactions List Of Combination Reactions A combination reaction is a reaction in which two or more substances combine to form a single new substance. 2h2 + o2 → 2h2o. The burning of carbon in the presence of oxygen to produce carbon dioxide is a combination reaction: Combination reactions describe a reaction like this: Combination reaction may occur between two elements, two compounds, or between a. List Of Combination Reactions.
From www.slideserve.com
PPT Chapter 5 Chemical Reactions PowerPoint Presentation, free List Of Combination Reactions 2h2 + o2 → 2h2o. The reactants may be elements or compounds, while the product is always a compound. Combination reaction may occur between two elements, two compounds, or between a compound and an element. A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions describe a reaction like. List Of Combination Reactions.