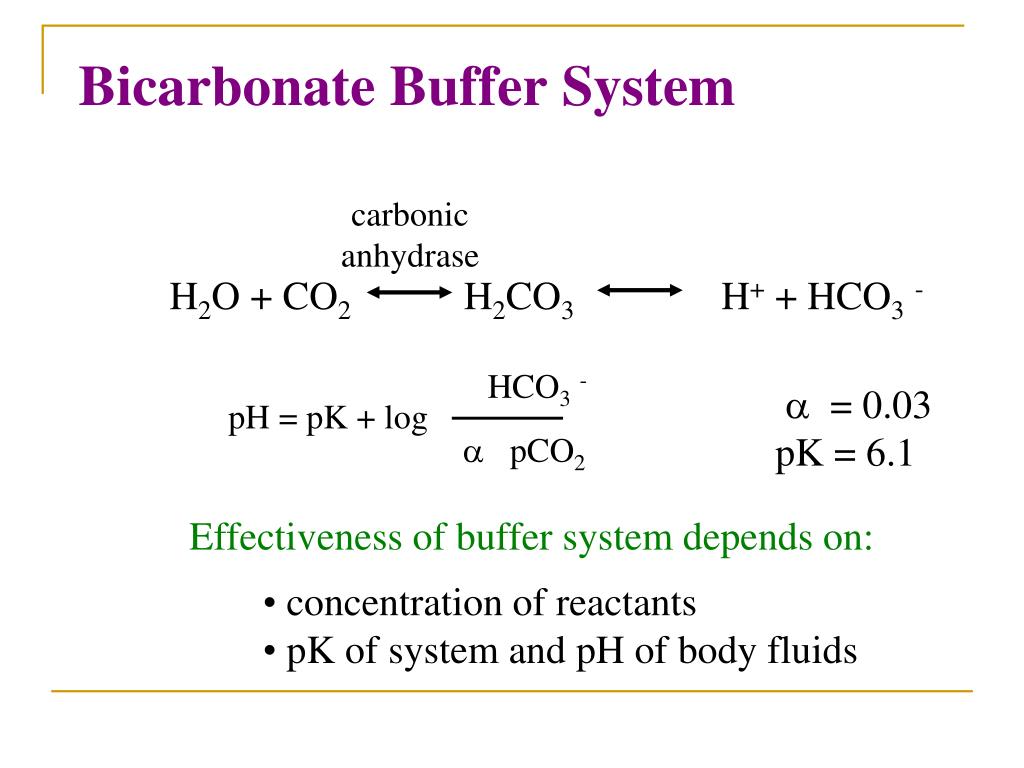
Why Is The Bicarbonate Buffer System Considered An Open Buffer System . The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. Firstly, it is the most plentiful buffer within the body; The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. Bicarbonate as the main extracellular buffer system. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The classical buffer describes a. Bicarbonate is an effective buffer because it is: Secondly, it acts as an open buffer system. The bicarbonate system is important for two reasons. Co 2 can be adjusted by changing. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid.
from www.slideserve.com
Co 2 can be adjusted by changing. Bicarbonate is an effective buffer because it is: With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. Firstly, it is the most plentiful buffer within the body; Bicarbonate as the main extracellular buffer system. The classical buffer describes a. The bicarbonate system is important for two reasons. Secondly, it acts as an open buffer system. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are.
PPT AcidBase Regulation PowerPoint Presentation, free download ID
Why Is The Bicarbonate Buffer System Considered An Open Buffer System Bicarbonate as the main extracellular buffer system. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. Bicarbonate is an effective buffer because it is: The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. Secondly, it acts as an open buffer system. Co 2 can be adjusted by changing. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Bicarbonate as the main extracellular buffer system. The bicarbonate system is important for two reasons. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The classical buffer describes a. Firstly, it is the most plentiful buffer within the body;
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. Co 2 can be adjusted by changing. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT AcidBase Regulation PowerPoint Presentation, free download ID Why Is The Bicarbonate Buffer System Considered An Open Buffer System Bicarbonate as the main extracellular buffer system. Co 2 can be adjusted by changing. Firstly, it is the most plentiful buffer within the body; The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Bicarbonate is an effective buffer because it is: The bicarbonate system is important for. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download Why Is The Bicarbonate Buffer System Considered An Open Buffer System With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Co 2 can be adjusted by changing. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. Bicarbonate as the main extracellular buffer system. The bicarbonate buffer system is a crucial physiological mechanism that helps. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT RESPIRATORY SYSTEM 3 PowerPoint Presentation, free download Why Is The Bicarbonate Buffer System Considered An Open Buffer System The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The bicarbonate system is important for two reasons. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. Firstly, it is the most plentiful buffer within the body; The bicarbonate. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Why Is The Bicarbonate Buffer System Considered An Open Buffer System Firstly, it is the most plentiful buffer within the body; With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate system is important for two reasons. Secondly, it acts as an open buffer system. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. The. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Lecture Goals PowerPoint Presentation, free download ID3309237 Why Is The Bicarbonate Buffer System Considered An Open Buffer System Firstly, it is the most plentiful buffer within the body; With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching Why Is The Bicarbonate Buffer System Considered An Open Buffer System Firstly, it is the most plentiful buffer within the body; Bicarbonate is an effective buffer because it is: The classical buffer describes a. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Biochemistry of acidobasic regulations PowerPoint Presentation Why Is The Bicarbonate Buffer System Considered An Open Buffer System Bicarbonate as the main extracellular buffer system. The bicarbonate system is important for two reasons. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer system is a crucial. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1562690 Why Is The Bicarbonate Buffer System Considered An Open Buffer System With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Secondly, it acts as an open buffer system. The bicarbonate system is important for two reasons. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The classical buffer describes a. Bicarbonate is an effective. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideshare.net
Buffer system Why Is The Bicarbonate Buffer System Considered An Open Buffer System Secondly, it acts as an open buffer system. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. The bicarbonate system is important for two reasons. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. With. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.numerade.com
Drag the labels onto the diagram to identify the components of the Why Is The Bicarbonate Buffer System Considered An Open Buffer System Bicarbonate is an effective buffer because it is: The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Firstly, it is the most plentiful buffer within the body; The bicarbonate buffer is. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT pH and Biological Systems PowerPoint Presentation, free download Why Is The Bicarbonate Buffer System Considered An Open Buffer System The classical buffer describes a. The bicarbonate system is important for two reasons. Secondly, it acts as an open buffer system. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. Co 2 can be adjusted by changing. With intact open buffer mechanisms, the bicarbonate system can accommodate vast. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.pinterest.com
(MCAT favorite) VHY Bicarbonate Buffer System Medical student Why Is The Bicarbonate Buffer System Considered An Open Buffer System Bicarbonate is an effective buffer because it is: The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. Bicarbonate as the main extracellular buffer system. The classical buffer describes a. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From quizparaguayan.z4.web.core.windows.net
How Bicarbonate Buffer System Works Why Is The Bicarbonate Buffer System Considered An Open Buffer System With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. Secondly, it acts as an open buffer system. The bicarbonate buffer is the primary buffering system of the if surrounding the cells. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download Why Is The Bicarbonate Buffer System Considered An Open Buffer System The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Bicarbonate is an effective buffer because it is: Secondly, it acts as an open buffer system. The. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From slideplayer.com
Chapter 9 Acids, Bases and Buffers in the Body ppt download Why Is The Bicarbonate Buffer System Considered An Open Buffer System The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. Bicarbonate as the main extracellular buffer system. The bicarbonate system is important for two reasons. Firstly, it is the most plentiful buffer within the body; Secondly, it acts as an open buffer system. The bicarbonate buffer is the primary. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From quizparaguayan.z4.web.core.windows.net
Bicarbonate Buffer Explained Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Firstly, it is the most plentiful buffer within the body; The bicarbonate buffer system is a crucial physiological mechanism that helps maintain. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Water Balance PowerPoint Presentation, free download ID2563527 Why Is The Bicarbonate Buffer System Considered An Open Buffer System Bicarbonate is an effective buffer because it is: Bicarbonate as the main extracellular buffer system. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate system is important for two reasons. The classical buffer describes a. Firstly, it is the most plentiful buffer within the body;. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.youtube.com
Transport and Exchange of Carbon Dioxide and Oxygen The Bicarbonate Why Is The Bicarbonate Buffer System Considered An Open Buffer System With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Firstly, it is the most plentiful buffer within the body; The classical buffer describes a. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The bicarbonate buffer system is a crucial mechanism that helps. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From quizlet.com
The bicarbonate buffer system Diagram Quizlet Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Secondly, it acts as an open buffer system. The bicarbonate system is important for two reasons. Bicarbonate is an effective buffer because it is: Bicarbonate as the main extracellular buffer system. Co 2 can be adjusted by changing.. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From lindsay-kbeard.blogspot.com
Describe a System Buffered With Carbonic Acid Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate system is important for two reasons. Co 2 can be adjusted by changing. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The bicarbonate buffer is the primary buffering system of the. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From quizdbwinemaster.z21.web.core.windows.net
What Is Bicarbonate Buffer System Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Bicarbonate is an effective buffer because it is: The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. Bicarbonate as the main extracellular buffer system. Firstly, it. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Why Is The Bicarbonate Buffer System Considered An Open Buffer System The classical buffer describes a. The bicarbonate system is important for two reasons. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Co 2 can be adjusted by changing. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Why Is The Bicarbonate Buffer System Considered An Open Buffer System Firstly, it is the most plentiful buffer within the body; Bicarbonate as the main extracellular buffer system. Co 2 can be adjusted by changing. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. The classical buffer describes a. The bicarbonate system is important for two reasons. The bicarbonate buffer system. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Why Is The Bicarbonate Buffer System Considered An Open Buffer System The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The classical buffer describes a. Secondly, it acts as an open buffer system. Co 2 can be adjusted by changing. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Why Is The Bicarbonate Buffer System Considered An Open Buffer System Secondly, it acts as an open buffer system. Co 2 can be adjusted by changing. The bicarbonate system is important for two reasons. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. Bicarbonate as the main extracellular buffer system. Bicarbonate is an effective buffer because. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate system is important for two reasons. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The classical buffer describes a. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer is the primary buffering system of the if surrounding. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation ID3224155 Why Is The Bicarbonate Buffer System Considered An Open Buffer System Secondly, it acts as an open buffer system. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. Bicarbonate as the main extracellular buffer system. Firstly, it. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Figure 10.9 The respiratory cycle. PowerPoint Presentation ID Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate system is important for two reasons. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Firstly, it is the most plentiful buffer within the. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From study.com
Bicarbonate Buffer System Overview, Equation & Uses Lesson Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is.. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.youtube.com
The bicarbonate buffer system YouTube Why Is The Bicarbonate Buffer System Considered An Open Buffer System The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are. Secondly, it acts as an open buffer system. Co 2 can be adjusted by changing. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is.. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideshare.net
Buffer system Why Is The Bicarbonate Buffer System Considered An Open Buffer System The bicarbonate system is important for two reasons. Firstly, it is the most plentiful buffer within the body; Bicarbonate is an effective buffer because it is: With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Buffer systems PowerPoint Presentation, free download ID9427698 Why Is The Bicarbonate Buffer System Considered An Open Buffer System The classical buffer describes a. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. Co 2 can be adjusted by changing. Secondly, it acts as an open buffer system. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.slideserve.com
PPT Chapter 27 PowerPoint Presentation, free download ID3705071 Why Is The Bicarbonate Buffer System Considered An Open Buffer System Secondly, it acts as an open buffer system. The bicarbonate buffer system is a crucial mechanism that helps maintain a stable ph in the body, typically between 7.35 and 7.45, which is. The bicarbonate system is important for two reasons. The classical buffer describes a. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Bicarbonate. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.
From www.researchgate.net
The model for lactic acid buffering by bicarbonate and the control of Why Is The Bicarbonate Buffer System Considered An Open Buffer System The classical buffer describes a. The bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout the body. With intact open buffer mechanisms, the bicarbonate system can accommodate vast quantities of acid. Firstly, it is the most plentiful buffer within the body; The bicarbonate buffer system is a crucial mechanism that helps maintain a. Why Is The Bicarbonate Buffer System Considered An Open Buffer System.