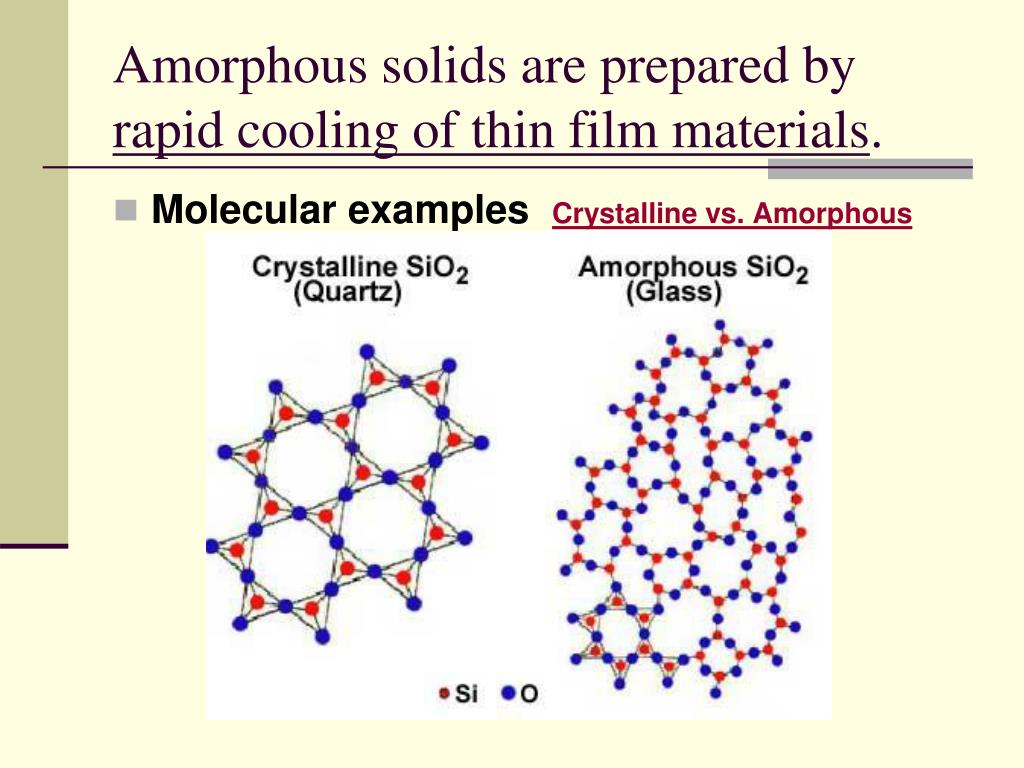
Amorphous Solid Glass Examples . An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Explain why materials form amorphous rather than crystalline solids. This leads to many fascinating. An amorphous solid is a solid that lacks an ordered internal structure. Compare crystalline and amorphous solids in terms of their composition,. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Examples of amorphous solids include glass, rubber, and. An amorphous, translucent solid is called a glass. Glass can be widely defined as an amorphous solid.
from www.slideserve.com
Compare crystalline and amorphous solids in terms of their composition,. Glass can be widely defined as an amorphous solid. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Explain why materials form amorphous rather than crystalline solids. An amorphous, translucent solid is called a glass. Examples of amorphous solids include glass, rubber, and. This leads to many fascinating. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. An amorphous solid is a solid that lacks an ordered internal structure.
PPT Liquids and Solids PowerPoint Presentation, free download ID
Amorphous Solid Glass Examples An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. This leads to many fascinating. Compare crystalline and amorphous solids in terms of their composition,. An amorphous solid is a solid that lacks an ordered internal structure. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Examples of amorphous solids include glass, rubber, and. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Glass can be widely defined as an amorphous solid. Explain why materials form amorphous rather than crystalline solids. An amorphous, translucent solid is called a glass.
From www.youtube.com
What is CRYSTALLINE & AMORPHOUS solid Basic PRACTICAL CHEMISTRY YouTube Amorphous Solid Glass Examples Glass can be widely defined as an amorphous solid. Examples of amorphous solids include glass, rubber, and. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Compare crystalline and amorphous solids in terms of their composition,. This leads to many fascinating. An amorphous solid is a solid that lacks an ordered internal structure.. Amorphous Solid Glass Examples.
From www.researchgate.net
Schematic representation of the structure of an amorphous solid in the Amorphous Solid Glass Examples Explain why materials form amorphous rather than crystalline solids. This leads to many fascinating. Examples of amorphous solids include glass, rubber, and. Glass can be widely defined as an amorphous solid. An amorphous, translucent solid is called a glass. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Almost any substance can solidify. Amorphous Solid Glass Examples.
From www.toppr.com
Crystalline and Amorphous Solids Explanation, Differences, Examples, etc Amorphous Solid Glass Examples An amorphous solid is a solid that lacks an ordered internal structure. An amorphous, translucent solid is called a glass. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Examples of amorphous solids include glass, rubber, and. Compare crystalline and amorphous solids in terms of their composition,. This leads to many fascinating. Almost. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT States of Matter Chp 3 Lecture 1 PowerPoint Presentation, free Amorphous Solid Glass Examples This leads to many fascinating. An amorphous solid is a solid that lacks an ordered internal structure. Compare crystalline and amorphous solids in terms of their composition,. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Explain why materials form amorphous rather than crystalline solids. Almost any substance can solidify in amorphous form. Amorphous Solid Glass Examples.
From www.shutterstock.com
Amorphous Crystal Glass Structure Difference Structure 库存矢量图(免版税 Amorphous Solid Glass Examples An amorphous, translucent solid is called a glass. This leads to many fascinating. Glass can be widely defined as an amorphous solid. Examples of amorphous solids include glass, rubber, and. Compare crystalline and amorphous solids in terms of their composition,. An amorphous solid is a solid that lacks an ordered internal structure. An amorphous solid can be considered to have. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Analysis of Glass Glass Evidence PowerPoint Presentation, free Amorphous Solid Glass Examples Glass can be widely defined as an amorphous solid. Explain why materials form amorphous rather than crystalline solids. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. This leads to many fascinating. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more.. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Chapter 3 & 4 Glass Evidence PowerPoint Presentation ID2260390 Amorphous Solid Glass Examples Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Explain why materials form amorphous rather than crystalline solids. Compare crystalline and amorphous solids in terms of their composition,. This leads to many fascinating. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. An amorphous solid can. Amorphous Solid Glass Examples.
From www.youtube.com
amorphous and crystalline solids YouTube Amorphous Solid Glass Examples An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Examples of amorphous solids include glass, rubber, and. Compare crystalline and amorphous solids in terms of their composition,. An amorphous, translucent solid is. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Forensic Science Analysis of Glass Evidence PowerPoint Amorphous Solid Glass Examples This leads to many fascinating. An amorphous, translucent solid is called a glass. Glass can be widely defined as an amorphous solid. Explain why materials form amorphous rather than crystalline solids. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. An amorphous solid is a solid that lacks. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Electrical Engineering Materials PowerPoint Presentation, free Amorphous Solid Glass Examples Explain why materials form amorphous rather than crystalline solids. An amorphous, translucent solid is called a glass. Compare crystalline and amorphous solids in terms of their composition,. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Glass can be widely defined as an amorphous solid. An amorphous solid. Amorphous Solid Glass Examples.
From eduinput.com
Amorphous Solids Definition, Properties, Examples, uses Amorphous Solid Glass Examples Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. An amorphous, translucent solid is called a glass. Compare crystalline and amorphous solids in terms of their composition,. Explain why materials form amorphous rather than crystalline solids. Glass can be widely defined as an amorphous solid. Almost any substance can solidify in amorphous form. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation, free download ID441958 Amorphous Solid Glass Examples Examples of amorphous solids include glass, rubber, and. An amorphous solid is a solid that lacks an ordered internal structure. This leads to many fascinating. Compare crystalline and amorphous solids in terms of their composition,. An amorphous, translucent solid is called a glass. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Explain. Amorphous Solid Glass Examples.
From www.youtube.com
Crystalline and amorphous Solids Crystallography Dr Ilham YouTube Amorphous Solid Glass Examples Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Glass can be widely defined as an amorphous solid. Examples of amorphous solids include glass, rubber, and. Compare crystalline and amorphous solids in terms of their composition,. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. An. Amorphous Solid Glass Examples.
From eduinput.com
Amorphous Solids Definition, Properties, Examples, uses Amorphous Solid Glass Examples An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. An amorphous, translucent solid is called a glass. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly. Amorphous Solid Glass Examples.
From physicsworld.com
Field theory of amorphous solids describes toothpaste, concrete and Amorphous Solid Glass Examples Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Examples of amorphous solids include glass, rubber, and. Explain why materials form amorphous rather than crystalline solids. This leads to many fascinating. Compare crystalline and amorphous solids in terms of their composition,. Glass can be widely defined as an amorphous solid. An amorphous solid. Amorphous Solid Glass Examples.
From chemistnotes.com
Crystalline solid and Amorphous Solid Chemistry Notes Amorphous Solid Glass Examples Explain why materials form amorphous rather than crystalline solids. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. An amorphous, translucent solid is called a glass. Examples of amorphous solids include glass,. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Forensic analysis of glass PowerPoint Presentation, free download Amorphous Solid Glass Examples Compare crystalline and amorphous solids in terms of their composition,. Explain why materials form amorphous rather than crystalline solids. Examples of amorphous solids include glass, rubber, and. This leads to many fascinating. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. An amorphous, translucent solid is called a glass. An amorphous solid is. Amorphous Solid Glass Examples.
From eduinput.com
Classification of Solids Crystalline Solids, Amorphous Solids, and Amorphous Solid Glass Examples Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. An amorphous solid is a solid that lacks an ordered internal structure. Explain why materials form amorphous rather than crystalline solids. Examples of amorphous solids include glass, rubber, and. This leads to many fascinating. Compare crystalline and amorphous solids in terms of their composition,.. Amorphous Solid Glass Examples.
From newscenter.lbl.gov
Scientists Achieve FirstEver 3D Atomic Imaging of an Amorphous Solid Amorphous Solid Glass Examples An amorphous, translucent solid is called a glass. Glass can be widely defined as an amorphous solid. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. An amorphous solid is a solid that lacks an ordered internal. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Amorphous Solid Glass Examples An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Compare crystalline and amorphous solids in terms of their composition,. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Examples of amorphous solids include glass, rubber, and. An amorphous solid is a. Amorphous Solid Glass Examples.
From testbook.com
Amorphous Solids Learn its Definition, Examples and Properties Amorphous Solid Glass Examples Compare crystalline and amorphous solids in terms of their composition,. Examples of amorphous solids include glass, rubber, and. Explain why materials form amorphous rather than crystalline solids. This leads to many fascinating. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Glass can be widely defined as an amorphous solid. Amorphous solids are. Amorphous Solid Glass Examples.
From chemistnotes.com
Crystalline solid and Amorphous Solid Chemistry Notes Amorphous Solid Glass Examples An amorphous solid is a solid that lacks an ordered internal structure. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. This leads to many fascinating. Examples of amorphous solids include glass, rubber, and. Glass can be widely defined as an amorphous solid. Compare crystalline and amorphous solids in terms of their composition,.. Amorphous Solid Glass Examples.
From webmis.highland.cc.il.us
Crystalline and Amorphous Solids Amorphous Solid Glass Examples This leads to many fascinating. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Compare crystalline and amorphous solids in terms of their composition,. Examples of amorphous solids include glass, rubber, and. An amorphous solid is a solid that lacks an ordered internal structure. An amorphous, translucent solid is called a glass. An. Amorphous Solid Glass Examples.
From study.com
Amorphous Solid Definition & Examples Video & Lesson Transcript Amorphous Solid Glass Examples Glass can be widely defined as an amorphous solid. Compare crystalline and amorphous solids in terms of their composition,. An amorphous, translucent solid is called a glass. Explain why materials form amorphous rather than crystalline solids. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Examples of amorphous solids include glass, rubber, and.. Amorphous Solid Glass Examples.
From u.osu.edu
Amorphous Materials Materials Science for High School Amorphous Solid Glass Examples Glass can be widely defined as an amorphous solid. An amorphous, translucent solid is called a glass. Explain why materials form amorphous rather than crystalline solids. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. This leads to many fascinating. Amorphous solids are solids that have no regular crystal pattern to their constituent. Amorphous Solid Glass Examples.
From collegedunia.com
Amorphous Solid Definition, Properties & Examples Amorphous Solid Glass Examples Compare crystalline and amorphous solids in terms of their composition,. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. This leads to many fascinating. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Glass can be widely defined as an amorphous. Amorphous Solid Glass Examples.
From chem.libretexts.org
12.1 Crystalline and Amorphous Solids Chemistry LibreTexts Amorphous Solid Glass Examples An amorphous solid is a solid that lacks an ordered internal structure. This leads to many fascinating. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Examples of amorphous solids include glass, rubber, and. Explain why materials form amorphous rather than crystalline solids. Glass can be widely defined. Amorphous Solid Glass Examples.
From sciencestruck.com
Crystalline Vs. Amorphous Solids What's the Difference? Amorphous Solid Glass Examples An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Compare crystalline and amorphous solids in terms of their composition,. Examples of amorphous solids include glass, rubber, and. An amorphous solid is a. Amorphous Solid Glass Examples.
From www.askiitians.com
The Solid State Study Material for IIT JEE askIITians Amorphous Solid Glass Examples Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Examples of amorphous solids include glass, rubber, and. Compare crystalline and amorphous solids in terms of their composition,. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Amorphous solids are solids that. Amorphous Solid Glass Examples.
From slideplayer.com
Solids. ppt download Amorphous Solid Glass Examples An amorphous solid is a solid that lacks an ordered internal structure. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Glass can be widely defined as an amorphous solid. Examples of amorphous solids include glass, rubber, and. An amorphous solid can be considered to have a random arrangement of atoms, such as. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Amorphous Solid Glass Examples Compare crystalline and amorphous solids in terms of their composition,. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Examples of amorphous solids include glass, rubber, and. An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. An amorphous, translucent solid is. Amorphous Solid Glass Examples.
From www.aniwaa.com
Thermoplastics for AM SemiCrystalline vs Amorphous Amorphous Solid Glass Examples Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Compare crystalline and amorphous solids in terms of their composition,. Glass can be widely defined as an amorphous solid. This leads to many fascinating. An amorphous, translucent solid is called a glass. An amorphous solid is a solid that lacks an ordered internal structure.. Amorphous Solid Glass Examples.
From eduinput.com
7 Difference between crystalline solids and amorphous solids Amorphous Solid Glass Examples Examples of amorphous solids include glass, rubber, and. Compare crystalline and amorphous solids in terms of their composition,. Explain why materials form amorphous rather than crystalline solids. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. An amorphous, translucent solid is called a glass. Glass can be widely defined as an amorphous solid.. Amorphous Solid Glass Examples.
From www.slideserve.com
PPT Solid Crystal Structures (based on Chap. 12 Sec 11 of Jespersen 6 Amorphous Solid Glass Examples An amorphous solid can be considered to have a random arrangement of atoms, such as observed in a gas, but more. Examples of amorphous solids include glass, rubber, and. An amorphous solid is a solid that lacks an ordered internal structure. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Explain why materials. Amorphous Solid Glass Examples.
From pediaa.com
Difference Between Amorphous and Crystalline Solids Definition Amorphous Solid Glass Examples Examples of amorphous solids include glass, rubber, and. Amorphous solids are solids that have no regular crystal pattern to their constituent atoms or molecules. Almost any substance can solidify in amorphous form if the liquid phase is cooled rapidly enough. Glass can be widely defined as an amorphous solid. This leads to many fascinating. An amorphous, translucent solid is called. Amorphous Solid Glass Examples.