Lead Chloride Reaction Equation . It is slightly soluble in cold water, but its solubility. You can think of lead (ii). Lead (ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead(ii) chloride is a white solid, melting at 501°c. It is a lead coordination entity and an inorganic chloride. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions.
from exoahnwtg.blob.core.windows.net
Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. You can think of lead (ii). This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead(ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water, but its solubility. Lead (ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. It is a lead coordination entity and an inorganic chloride.
Lead Hydroxide And Hydrogen Chloride at Patsy Hines blog
Lead Chloride Reaction Equation Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. It is a lead coordination entity and an inorganic chloride. You can think of lead (ii). Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead (ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. Lead(ii) chloride is a white solid, melting at 501°c. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. It is slightly soluble in cold water, but its solubility.
From www.numerade.com
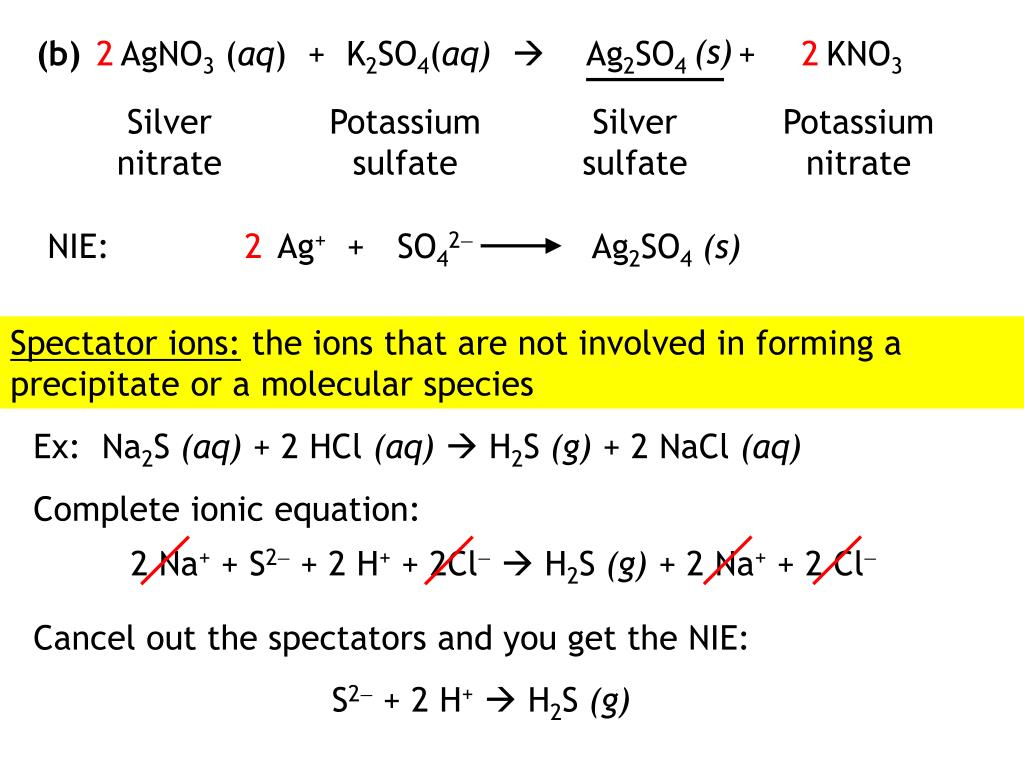
SOLVEDAqueous aluminum chloride reacts with a lead (II) nitrate Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. It is a lead coordination entity and an inorganic chloride. Lead(ii) chloride is a white solid, melting at 501°c. Lead (ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water,. Lead Chloride Reaction Equation.
From exoahnwtg.blob.core.windows.net
Lead Hydroxide And Hydrogen Chloride at Patsy Hines blog Lead Chloride Reaction Equation Lead(ii) chloride is a white solid, melting at 501°c. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. It is very slightly soluble in cold water, but more soluble in hot water. It is slightly soluble in cold water, but its solubility. Lead (ii) chloride is a white solid,. Lead Chloride Reaction Equation.
From klaiketkj.blob.core.windows.net
Aluminum Chloride Reacts With Lead Ii Nitrate at Becky Warren blog Lead Chloride Reaction Equation It is slightly soluble in cold water, but its solubility. Lead(ii) chloride is a white solid, melting at 501°c. Lead (ii) chloride is a white solid, melting at 501°c. The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead(ii) chloride, a white precipitate, is formed. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVED Sodium chloride and lead(II) acetate. Express your answer as a Lead Chloride Reaction Equation This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. You can think of lead (ii). Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. Lead (ii) chloride is a white solid, melting at 501°c. It is slightly soluble in. Lead Chloride Reaction Equation.
From tiannagroharvey.blogspot.com
Aqueous Sodium Chloride Reacts With Aqueous Lead Ii Nitrate Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead(ii) chloride is a white solid, melting at 501°c. It is slightly soluble in cold water, but its solubility. It is very slightly soluble in cold water, but more soluble in hot water. Lead(ii) chloride, a. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. It is a lead coordination entity and an inorganic chloride. Lead(ii) chloride is a white solid, melting at 501°c. You can think of lead (ii). It is very slightly soluble in cold water, but more soluble. Lead Chloride Reaction Equation.
From www.slideserve.com
PPT Solubility Rules PowerPoint Presentation, free download ID4934641 Lead Chloride Reaction Equation Lead (ii) chloride is a white solid, melting at 501°c. It is a lead coordination entity and an inorganic chloride. It is slightly soluble in cold water, but its solubility. The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. You can think of lead (ii).. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Lead Chloride Reaction Equation It is slightly soluble in cold water, but its solubility. Lead(ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. Lead(ii) chloride, a white precipitate, is formed by. Lead Chloride Reaction Equation.
From exoahnwtg.blob.core.windows.net
Lead Hydroxide And Hydrogen Chloride at Patsy Hines blog Lead Chloride Reaction Equation Lead (ii) chloride is a white solid, melting at 501°c. You can think of lead (ii). It is slightly soluble in cold water, but its solubility. Lead(ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. Lead (ii) chloride is an inorganic chloride consisting of two chlorine. Lead Chloride Reaction Equation.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Chloride Reaction Equation Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It is a lead coordination entity and an inorganic chloride. Lead(ii) chloride is a white solid, melting at 501°c. It. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVEDWrite the net ionic equation for the dissolution of lead(Il Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead(ii) chloride is a white solid, melting at 501°c. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. Lead(ii) chloride, a white precipitate, is formed by. Lead Chloride Reaction Equation.
From byjus.com
36. How many mole of lead(II) chloride will be formed from a reaction Lead Chloride Reaction Equation Lead (ii) chloride is a white solid, melting at 501°c. Lead(ii) chloride is a white solid, melting at 501°c. The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central. Lead Chloride Reaction Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. It is slightly soluble in cold water, but its solubility. Lead(ii) chloride is a white solid, melting at 501°c. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVED Write complete ionic equation and nct ionic equation solution Lead Chloride Reaction Equation You can think of lead (ii). It is slightly soluble in cold water, but its solubility. It is a lead coordination entity and an inorganic chloride. Lead(ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. The chemical formula for lead chloride is pbcl 2 which is. Lead Chloride Reaction Equation.
From en.ppt-online.org
Electrolysis online presentation Lead Chloride Reaction Equation It is a lead coordination entity and an inorganic chloride. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. Lead(ii) chloride is a white solid, melting at 501°c. The chemical. Lead Chloride Reaction Equation.
From brainly.in
Equation of reaction between lead and copper chloride via displacement Lead Chloride Reaction Equation It is a lead coordination entity and an inorganic chloride. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) chloride is a white solid, melting at 501°c. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The. Lead Chloride Reaction Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NaCl + Zn(NO3)2 = ZnCl2 + NaNO3 Lead Chloride Reaction Equation Lead(ii) chloride is a white solid, melting at 501°c. It is a lead coordination entity and an inorganic chloride. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. It is very slightly soluble in cold water, but more soluble in hot water. This page looks at the formation of some. Lead Chloride Reaction Equation.
From www.chegg.com
Solved If 1 mole of lead (II) nitrate reacts with 1 mole of Lead Chloride Reaction Equation Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. It is a lead coordination entity and an inorganic chloride. It is very slightly soluble in cold water, but more soluble in hot water. You can think of lead (ii). Lead (ii) chloride is a white solid, melting at 501°c. Lead(ii). Lead Chloride Reaction Equation.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Chloride Reaction Equation It is very slightly soluble in cold water, but more soluble in hot water. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. You can think of lead (ii). Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. It. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead Chloride Reaction Equation This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. It is slightly soluble in cold water, but its solubility. You can think of lead (ii).. Lead Chloride Reaction Equation.
From www.chegg.com
Solved When sodium chloride and lead (II) nitrate react in Lead Chloride Reaction Equation This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It is slightly soluble in cold water, but its solubility. It is a lead coordination entity and an inorganic chloride. You can think of lead (ii). Lead(ii) chloride is a white solid, melting at 501°c. It is very slightly soluble. Lead Chloride Reaction Equation.
From www.solutionspile.com
[Solved] The equation for the dissolving of lead (II) Lead Chloride Reaction Equation Lead(ii) chloride is a white solid, melting at 501°c. Lead (ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. It is slightly soluble in cold water, but its solubility. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Chloride Reaction Equation.
From www.toppr.com
Lead II Chloride Formula Definition, Concepts and Examples Lead Chloride Reaction Equation Lead (ii) chloride is a white solid, melting at 501°c. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. It is very slightly soluble in cold water, but more soluble in hot water. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVEDEnter a molecular equation for the precipitation reaction that Lead Chloride Reaction Equation This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It is very slightly soluble in cold water, but more soluble in hot water. It is slightly soluble in cold water, but its solubility. It is a lead coordination entity and an inorganic chloride. The chemical formula for lead chloride. Lead Chloride Reaction Equation.
From www.showme.com
ShowMe net ionic equation Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. You can think of lead (ii). It is slightly soluble in cold water, but its solubility. It is very slightly soluble in cold water, but more soluble in hot water. Lead (ii) chloride is a white. Lead Chloride Reaction Equation.
From www.tessshebaylo.com
Chemical Equation For Water And Sodium Chloride Tessshebaylo Lead Chloride Reaction Equation This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead(ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in hot water. It is a lead coordination entity and an inorganic chloride. Lead (ii) chloride is an inorganic chloride. Lead Chloride Reaction Equation.
From www.slideserve.com
PPT PRECIPITATION REACTIONS Solubility of Salts Section 18.4 Lead Chloride Reaction Equation Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. You can think of lead (ii). It is slightly soluble in cold water, but its solubility. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Lead (ii) chloride is. Lead Chloride Reaction Equation.
From www.slideserve.com
PPT Number One Sodium chloride solution + lead (II) acetate solutions Lead Chloride Reaction Equation Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Lead(ii) chloride is a white solid, melting at 501°c. It is very slightly soluble in cold water, but more soluble in. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVED points) Lead (II) chloride is insoluble In aqueous solution Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. It is very slightly soluble in cold water, but more soluble in hot water. It is slightly soluble in cold water, but its solubility. This page looks at the formation of some insoluble lead (ii) compounds. Lead Chloride Reaction Equation.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead Chloride Reaction Equation The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride. Lead(ii) chloride is a white solid, melting at 501°c. Lead (ii) chloride is a white solid, melting at 501°c. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central. Lead Chloride Reaction Equation.
From www.toppr.com
5. Write a balanced chemical equations the following chemical reactions Lead Chloride Reaction Equation Lead (ii) chloride is a white solid, melting at 501°c. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It is a lead coordination entity and an inorganic chloride. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution.. Lead Chloride Reaction Equation.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Lead Chloride Reaction Equation Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. It is slightly soluble in cold water, but its solubility. You can think of lead (ii). The chemical formula for lead chloride is pbcl 2 which is commonly known as lead chloride but its iupac name is lead (ll) chloride.. Lead Chloride Reaction Equation.
From www.chegg.com
Solved Solid lead(II) sulfide reacts with aqueous Lead Chloride Reaction Equation It is a lead coordination entity and an inorganic chloride. It is slightly soluble in cold water, but its solubility. Lead (ii) chloride is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. It is very slightly soluble in cold water, but more soluble in hot water. Lead (ii) chloride is a white solid,. Lead Chloride Reaction Equation.
From testbook.com
Lead (II) Chloride Formula Structure, Properties, Uses & More Lead Chloride Reaction Equation It is slightly soluble in cold water, but its solubility. It is very slightly soluble in cold water, but more soluble in hot water. You can think of lead (ii). Lead (ii) chloride is a white solid, melting at 501°c. It is a lead coordination entity and an inorganic chloride. Lead (ii) chloride is an inorganic chloride consisting of two. Lead Chloride Reaction Equation.
From www.youtube.com
How to write chemical formula Lead IV ChlorideMolecular formula Lead Lead Chloride Reaction Equation Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. It is slightly soluble in cold water, but its solubility. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It is very slightly soluble in cold water, but more. Lead Chloride Reaction Equation.