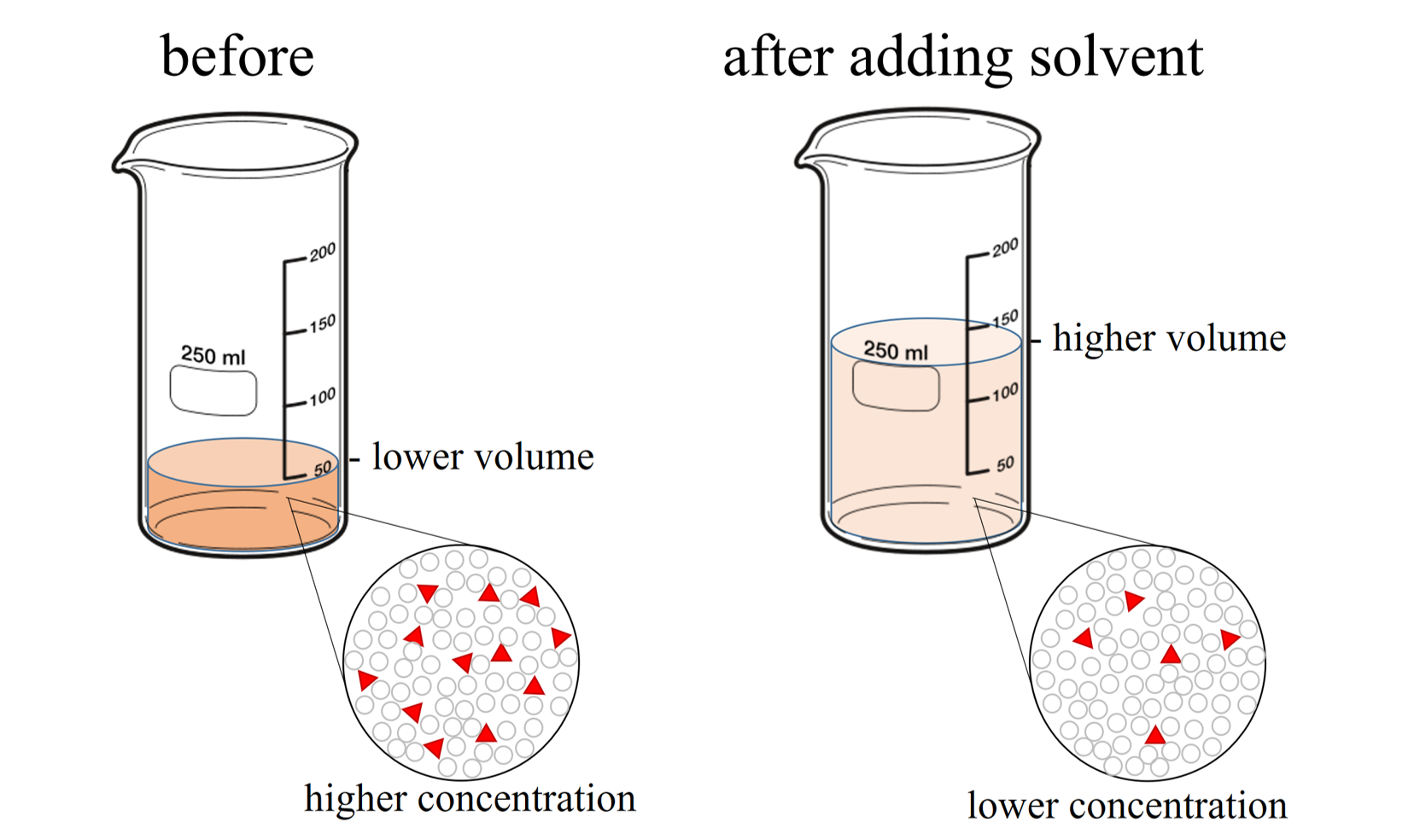
Dilute Solution Facts . For example, we might say that a glass. It affects reactions, creates standard solutions, and. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to accurately dilute solutions with this simple guide. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent.
from chem.libretexts.org
Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. A concentrated solution contains a. Learn how to accurately dilute solutions with this simple guide. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. For example, we might say that a glass. It affects reactions, creates standard solutions, and. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent.
14.7 Solution Dilution Chemistry LibreTexts
Dilute Solution Facts It affects reactions, creates standard solutions, and. Learn how to accurately dilute solutions with this simple guide. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. A concentrated solution contains a. For example, we might say that a glass. It affects reactions, creates standard solutions, and. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more solvent.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Solution Facts A concentrated solution contains a. Dilution is a process used to lower the concentration of the original solution by adding more solvent. It affects reactions, creates standard solutions, and. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy.. Dilute Solution Facts.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID Dilute Solution Facts For example, we might say that a glass. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A concentrated solution contains a. It affects reactions, creates standard solutions, and. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a crucial process in. Dilute Solution Facts.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to accurately dilute solutions with this simple guide. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. A. Dilute Solution Facts.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Solution Facts Learn how to accurately dilute solutions with this simple guide. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass. Dilution is a process used to lower the concentration of the. Dilute Solution Facts.
From stock.adobe.com
Solubility vector illustration. Labeled solute, solvent and solution Dilute Solution Facts Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. It affects reactions, creates standard solutions, and. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution.. Dilute Solution Facts.
From www.slideserve.com
PPT Solutions Part II PowerPoint Presentation, free download ID4272422 Dilute Solution Facts A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to accurately dilute solutions with this simple guide. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is a process used to lower the concentration of the. Dilute Solution Facts.
From www.haikudeck.com
Mixtures And Solutions by Alexandra Stearns Dilute Solution Facts A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. For example, we might say that a glass. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. It affects reactions, creates standard solutions, and. A concentrated solution contains a. Dilution is. Dilute Solution Facts.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilute Solution Facts Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. State whether the concentration of a solution is directly or indirectly proportional to its volume. A concentrated solution contains a. Learn how to accurately dilute solutions with this simple guide. Dilution is a process used to lower the concentration of the original solution by adding more. Dilute Solution Facts.
From www.slideserve.com
PPT Volumetric Analysis PowerPoint Presentation, free download ID Dilute Solution Facts A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. It affects reactions, creates standard solutions, and. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute. Dilute Solution Facts.
From www.showme.com
Concentrated vs. Diluted Solution Science ShowMe Dilute Solution Facts Learn how to accurately dilute solutions with this simple guide. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which the amount of solute (the substance that is dissolved). Dilute Solution Facts.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilute Solution Facts A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Dilution is a process used to lower the concentration of the original solution by adding more solvent. It affects reactions, creates standard solutions, and. For example, we might say that a glass. Learn how to accurately. Dilute Solution Facts.
From www.slideserve.com
PPT What is osmosis? PowerPoint Presentation, free download ID2712925 Dilute Solution Facts A concentrated solution contains a. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which. Dilute Solution Facts.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to accurately dilute solutions with this simple guide. A dilute solution is one in which there is a relatively small amount. Dilute Solution Facts.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3908272 Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. It affects reactions, creates standard solutions, and. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A concentrated solution contains a. For example, we might say that a glass. State whether the concentration. Dilute Solution Facts.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID528392 Dilute Solution Facts Learn how to accurately dilute solutions with this simple guide. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A concentrated solution contains a. It affects reactions,. Dilute Solution Facts.
From www.medicine.mcgill.ca
Serial Dilutions Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of.. Dilute Solution Facts.
From www.slideserve.com
PPT Mixtures, Solutions, and Water PowerPoint Presentation, free Dilute Solution Facts State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. It affects reactions, creates standard solutions, and. Dilution is a process used to lower. Dilute Solution Facts.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilute Solution Facts For example, we might say that a glass. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. It affects reactions, creates standard solutions, and. A dilute solution is one in which the amount of solute (the substance. Dilute Solution Facts.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Solution Facts Learn how to accurately dilute solutions with this simple guide. It affects reactions, creates standard solutions, and. A concentrated solution contains a. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. A dilute solution is one in which there is a relatively small amount of. Dilute Solution Facts.
From slideplayer.com
Chapter 22 Solutions Lesson ppt download Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. For example, we might say that a glass. It affects reactions, creates standard solutions, and. Learn how to accurately dilute solutions with this simple guide. A dilute solution is. Dilute Solution Facts.
From slideplayer.com
Solutions Chapter ppt download Dilute Solution Facts A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Learn how to accurately dilute solutions with this simple guide. A dilute solution is one in which the amount of solute (the substance that. Dilute Solution Facts.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Solution Facts It affects reactions, creates standard solutions, and. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Learn how to accurately dilute solutions with. Dilute Solution Facts.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Solution Facts Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. It affects reactions, creates standard solutions, and. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. A concentrated solution contains a. A dilute solution is one in which there is a relatively small amount of solute dissolved in. Dilute Solution Facts.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. A concentrated solution contains a. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that. Dilute Solution Facts.
From www.slideserve.com
PPT Chapter 16 Solutions PowerPoint Presentation, free download ID Dilute Solution Facts Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. Learn how to accurately dilute solutions with this simple guide. Dilution is a process used to lower the concentration of the original solution by adding more solvent. For example, we might say that a glass. A concentrated solution contains a. A dilute solution is one in. Dilute Solution Facts.
From byjus.com
What are the major differences between dilute and concentrated acids? Dilute Solution Facts A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. It affects reactions, creates standard solutions, and. Learn how to accurately dilute solutions with this simple guide. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution.. Dilute Solution Facts.
From www.slideserve.com
PPT Molarity & Dilutions PowerPoint Presentation, free download ID Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. A concentrated solution contains a. Learn how to accurately dilute solutions with this simple guide. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A dilute solution is one in which the amount. Dilute Solution Facts.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount. Dilute Solution Facts.
From www.youtube.com
TYPES OF SOLUTION,Dilution of Solution,Concentrated,Dilute solutions Dilute Solution Facts A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. It affects reactions, creates standard solutions, and. Learn how to accurately dilute solutions with this simple guide. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process whereby the concentration of a. Dilute Solution Facts.
From www.coursehero.com
[Solved] . Model 2 Chemical Solutions Dilute Solution Concentrated Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. For example, we might say that a glass. A dilute. Dilute Solution Facts.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Solution Facts A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. State whether the concentration of a solution is directly or indirectly proportional to its volume. For example,. Dilute Solution Facts.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of Dilute Solution Facts A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. A concentrated solution contains a. For example, we might say that a glass. Dilution is a crucial. Dilute Solution Facts.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilute Solution Facts Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is a crucial process in chemistry, used in labs, pharmaceuticals, and even homeopathy. For example, we might say that a glass. Dilution is. Dilute Solution Facts.
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Dilute Solution Facts State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is a process used to lower the concentration of the original solution by adding more solvent. For example, we might say that a glass. It affects reactions, creates standard solutions, and. A dilute solution is one in which the amount of solute (the substance. Dilute Solution Facts.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Facts State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. A concentrated solution contains a. For example, we might say that a glass. A dilute solution is one in which the amount of solute (the substance that is. Dilute Solution Facts.