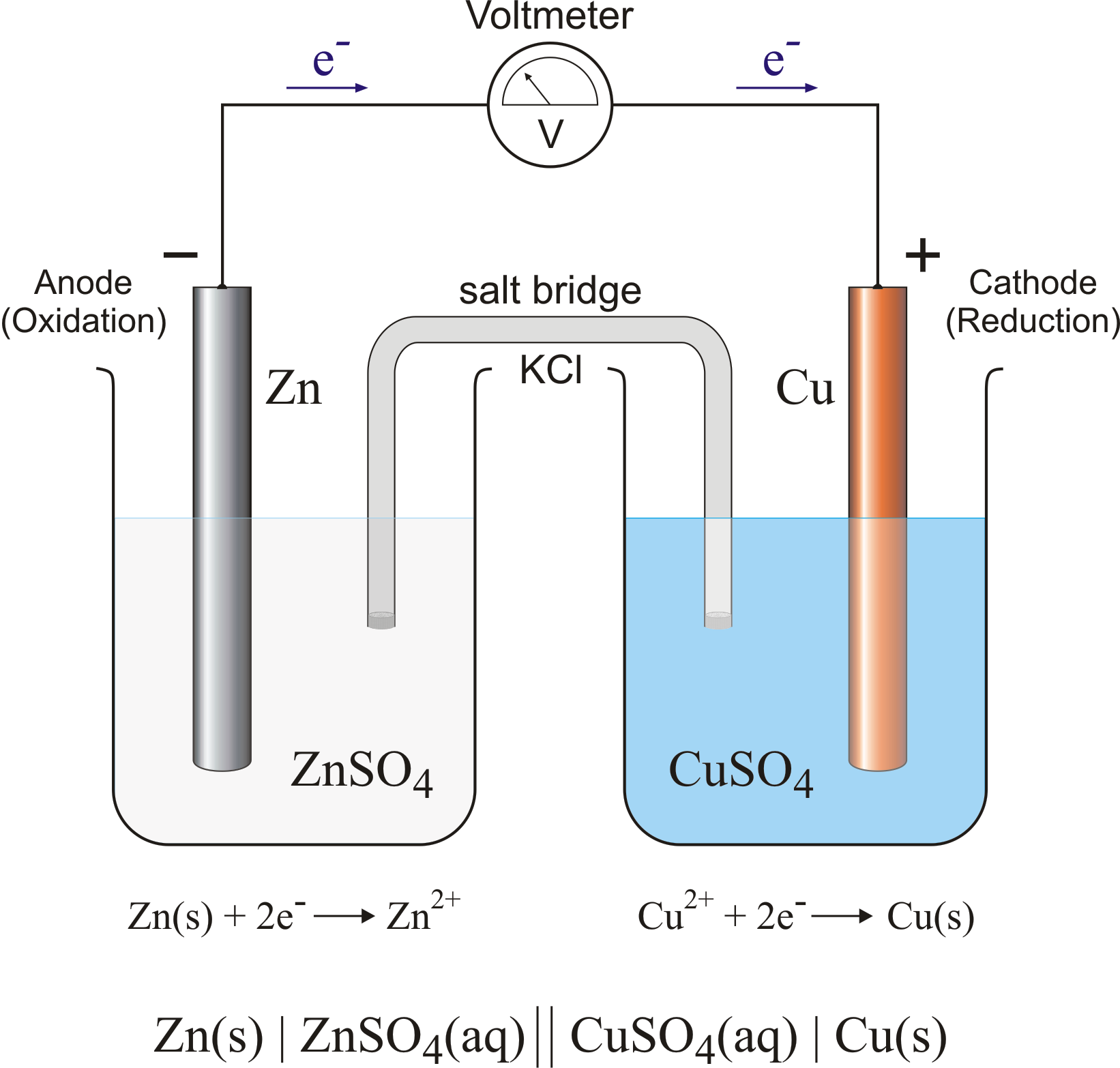
Zinc And Magnesium Galvanic Cell . Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The zinc half cell has a strip of zinc metal in a. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A galvanic cell contains two compartments: Describe the function of a galvanic cell and its components. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. By the end of this section, you will be able to: The picture opposite shows a galvanic cell made from zinc and tin half cells.
from glossary.periodni.com
The zinc half cell has a strip of zinc metal in a. Describe the function of a galvanic cell and its components. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. By the end of this section, you will be able to: One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to.
Galvanic cell Chemistry Dictionary & Glossary
Zinc And Magnesium Galvanic Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. By the end of this section, you will be able to: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Describe the function of a galvanic cell and its components. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The picture opposite shows a galvanic cell made from zinc and tin half cells. The zinc half cell has a strip of zinc metal in a. A galvanic cell contains two compartments:
From facts.net
9 Enigmatic Facts About Galvanic Cell Zinc And Magnesium Galvanic Cell The picture opposite shows a galvanic cell made from zinc and tin half cells. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A galvanic cell contains two compartments: Describe the function of a galvanic cell and its components. Use cell notation to describe the galvanic cell. Zinc And Magnesium Galvanic Cell.
From www.youtube.com
How To Draw Galvanic Cells and Voltaic Cells Electrochemistry YouTube Zinc And Magnesium Galvanic Cell The picture opposite shows a galvanic cell made from zinc and tin half cells. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Use cell notation to describe the galvanic cell where copper(ii) ions are. Zinc And Magnesium Galvanic Cell.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Zinc And Magnesium Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Describe the function of a galvanic cell and its components. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A galvanic cell contains two compartments:. Zinc And Magnesium Galvanic Cell.
From www.differencebetween.com
Difference Between Galvanic Cell and Concentration Cell Compare the Zinc And Magnesium Galvanic Cell Describe the function of a galvanic cell and its components. A galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal.. Zinc And Magnesium Galvanic Cell.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Zinc And Magnesium Galvanic Cell A galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Describe the function of a galvanic cell and its components. A typical cell might consist of two pieces of. Zinc And Magnesium Galvanic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Zinc And Magnesium Galvanic Cell A galvanic cell contains two compartments: The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. The zinc half cell has a strip of zinc metal in a. The picture opposite shows a galvanic cell made from zinc and tin half cells. One reactant gives up electrons (undergoes oxidation). Zinc And Magnesium Galvanic Cell.
From ar.inspiredpencil.com
Galvanic Cell Labeled Zinc And Magnesium Galvanic Cell A galvanic cell contains two compartments: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The zinc half cell has a strip of zinc metal in a. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is. Zinc And Magnesium Galvanic Cell.
From wps.prenhall.com
Media Portfolio Zinc And Magnesium Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The zinc half cell has a strip of zinc metal in a. Describe the function of a galvanic cell and its components. A galvanic cell contains two compartments: The two solutions are separated by a porous barrier that. Zinc And Magnesium Galvanic Cell.
From www.pinterest.com
Discover the Fascinating World of Galvanic Cells Zinc And Magnesium Galvanic Cell The zinc half cell has a strip of zinc metal in a. A galvanic cell contains two compartments: One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. By the end of this section, you will be able to: The picture opposite shows a galvanic cell made from zinc and tin half cells. Use cell notation to describe. Zinc And Magnesium Galvanic Cell.
From pediaa.com
Difference Between Daniell Cell and Galvanic Cell Definition, How Zinc And Magnesium Galvanic Cell Describe the function of a galvanic cell and its components. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. By the end of this section, you. Zinc And Magnesium Galvanic Cell.
From www.alamy.com
Daniell element galvanic cell with zinc and copper Stock Photo Zinc And Magnesium Galvanic Cell By the end of this section, you will be able to: The picture opposite shows a galvanic cell made from zinc and tin half cells. A galvanic cell contains two compartments: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. One reactant gives up electrons (undergoes oxidation). Zinc And Magnesium Galvanic Cell.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram Zinc And Magnesium Galvanic Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. The picture opposite shows a galvanic cell made from zinc and tin half cells. The zinc half cell has a strip of zinc metal in a. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced. Zinc And Magnesium Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells General Chemistry Zinc And Magnesium Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Describe the function of a galvanic cell and its components. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The zinc half cell has a strip of zinc metal in a. The picture opposite shows. Zinc And Magnesium Galvanic Cell.
From www.youtube.com
galvanic cell animation Zinc + silver YouTube Zinc And Magnesium Galvanic Cell The zinc half cell has a strip of zinc metal in a. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. One reactant gives up electrons. Zinc And Magnesium Galvanic Cell.
From www.slideserve.com
PPT Electrochemistry Part II The Galvanic Cell PowerPoint Zinc And Magnesium Galvanic Cell The zinc half cell has a strip of zinc metal in a. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Use cell notation to describe. Zinc And Magnesium Galvanic Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Magnesium Galvanic Cell Describe the function of a galvanic cell and its components. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. Zinc And Magnesium Galvanic Cell.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Zinc And Magnesium Galvanic Cell The zinc half cell has a strip of zinc metal in a. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A galvanic cell contains two. Zinc And Magnesium Galvanic Cell.
From www.chemistrylearner.com
Uncategorized Chemistry Learner Zinc And Magnesium Galvanic Cell The zinc half cell has a strip of zinc metal in a. The picture opposite shows a galvanic cell made from zinc and tin half cells. A galvanic cell contains two compartments: A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the. Zinc And Magnesium Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Zinc And Magnesium Galvanic Cell The zinc half cell has a strip of zinc metal in a. The picture opposite shows a galvanic cell made from zinc and tin half cells. By the end of this section, you will be able to: One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Use cell notation to describe the galvanic cell where copper(ii) ions. Zinc And Magnesium Galvanic Cell.
From byjus.com
in case of galvanic cell, it is said that zinc goes into the solution Zinc And Magnesium Galvanic Cell A galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. By the end of this section, you will be able to: The zinc half cell has a strip. Zinc And Magnesium Galvanic Cell.
From mungfali.com
Galvanic Cell Diagram Zinc And Magnesium Galvanic Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Describe the function of a galvanic cell and its components. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A galvanic cell contains two compartments:. Zinc And Magnesium Galvanic Cell.
From www.researchgate.net
Hector PEREZ Doctor of Philosophy Engineering University of Zinc And Magnesium Galvanic Cell A galvanic cell contains two compartments: Describe the function of a galvanic cell and its components. By the end of this section, you will be able to: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Use cell notation to describe the galvanic cell where copper(ii) ions. Zinc And Magnesium Galvanic Cell.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Zinc And Magnesium Galvanic Cell The picture opposite shows a galvanic cell made from zinc and tin half cells. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The zinc half cell has a strip of zinc metal in a.. Zinc And Magnesium Galvanic Cell.
From mavink.com
Zinc Copper Galvanic Cell Zinc And Magnesium Galvanic Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A galvanic cell contains two compartments: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Use cell notation to describe the galvanic cell where copper(ii) ions. Zinc And Magnesium Galvanic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Zinc And Magnesium Galvanic Cell By the end of this section, you will be able to: The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Describe the function of a galvanic cell and its components. The zinc half cell has a strip of zinc metal in a. One reactant gives up electrons (undergoes. Zinc And Magnesium Galvanic Cell.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 110 Zinc And Magnesium Galvanic Cell A galvanic cell contains two compartments: The zinc half cell has a strip of zinc metal in a. By the end of this section, you will be able to: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. A typical cell might consist of two pieces of. Zinc And Magnesium Galvanic Cell.
From www.vrogue.co
Chapter 3 Electrochemistry Galvanic Cell Part 1 Youtu vrogue.co Zinc And Magnesium Galvanic Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The zinc half cell has a strip of zinc metal in a. A galvanic cell contains two compartments: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The two solutions are separated by a porous. Zinc And Magnesium Galvanic Cell.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Zinc And Magnesium Galvanic Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. By the end of this section, you will be able to: A galvanic cell contains two compartments: The two solutions are separated by a porous barrier that prevents them from. Zinc And Magnesium Galvanic Cell.
From chem.libretexts.org
6.5 Standard Reduction Potentials Chemistry LibreTexts Zinc And Magnesium Galvanic Cell By the end of this section, you will be able to: The picture opposite shows a galvanic cell made from zinc and tin half cells. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. Use cell. Zinc And Magnesium Galvanic Cell.
From www.numerade.com
SOLVED Draw a galvanic cell between zinc and magnesium. Calculate the Zinc And Magnesium Galvanic Cell By the end of this section, you will be able to: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The zinc half cell has a strip of zinc metal in a. A typical cell might consist of two pieces of metal, one zinc and the other. Zinc And Magnesium Galvanic Cell.
From www.vrogue.co
Solved With The Aid Of A Clearly Labelled Diagram Des vrogue.co Zinc And Magnesium Galvanic Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. The two solutions are separated by a porous. Zinc And Magnesium Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Zinc And Magnesium Galvanic Cell The zinc half cell has a strip of zinc metal in a. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of. Zinc And Magnesium Galvanic Cell.
From saylordotorg.github.io
Describing Electrochemical Cells Zinc And Magnesium Galvanic Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. By the end of this section, you will be able to: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Use cell notation to describe the. Zinc And Magnesium Galvanic Cell.
From ar.inspiredpencil.com
Copper Silver Galvanic Cell Zinc And Magnesium Galvanic Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. The zinc half cell has a strip of zinc metal in a. By the end of this section, you will be able to: Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal. Zinc And Magnesium Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Zinc And Magnesium Galvanic Cell By the end of this section, you will be able to: A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Describe the function of a galvanic cell and its components. The zinc half cell has a strip of zinc. Zinc And Magnesium Galvanic Cell.