Iron Iii Chloride + Sodium Hydroxide . one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a precipitation reaction between iron (iii) chloride and sodium. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. there are three main steps for writing the net ionic equation for fecl3 +.
from www.numerade.com
a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). a precipitation reaction between iron (iii) chloride and sodium. there are three main steps for writing the net ionic equation for fecl3 +. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in.
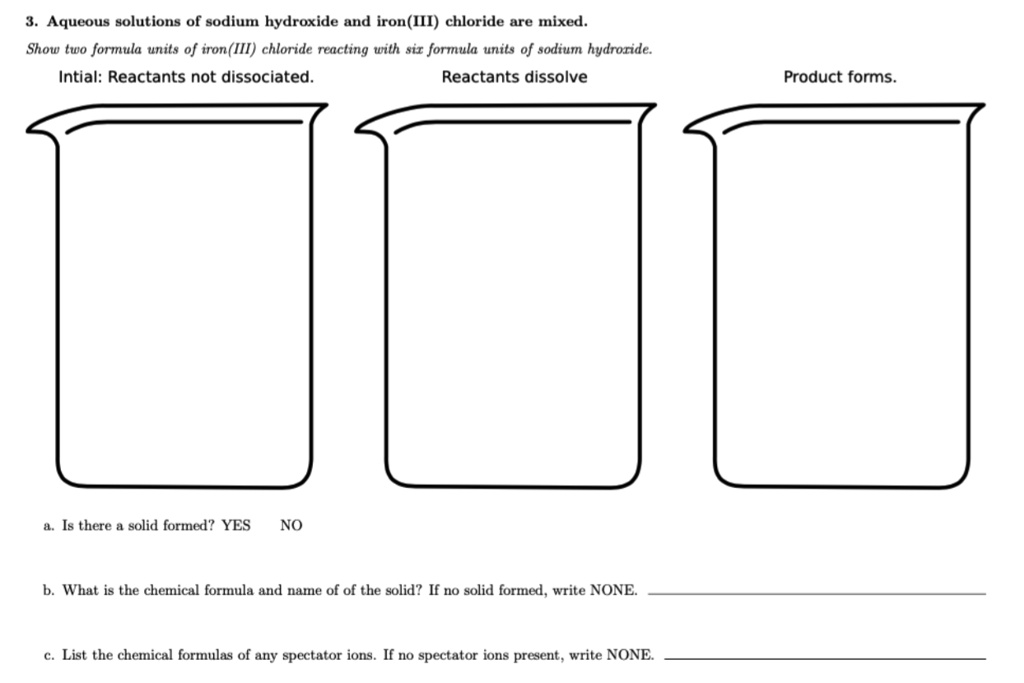
SOLVED Aqueous solutions of sodium hydroxide and iron(II) chloride are mixed Show two formula
Iron Iii Chloride + Sodium Hydroxide a precipitation reaction between iron (iii) chloride and sodium. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. there are three main steps for writing the net ionic equation for fecl3 +. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. a precipitation reaction between iron (iii) chloride and sodium.
From www.chegg.com
Solved 1. Consider the following reaction "Aqueous Iron Iii Chloride + Sodium Hydroxide one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. there are three main steps for writing the net ionic equation for fecl3 +. a solution of iron(iii)chloride is mixed. Iron Iii Chloride + Sodium Hydroxide.
From www.chegg.com
Solved Report Sheet Iron(III) Chloride + Sodium Hydroxide Iron Iii Chloride + Sodium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. there are three main steps for writing the net ionic equation for fecl3 +. one mole of ferric chloride [fecl 3], three moles of. Iron Iii Chloride + Sodium Hydroxide.
From www.youtube.com
The Reaction Between Iron (III) Nitrate and Sodium Hydroxide YouTube Iron Iii Chloride + Sodium Hydroxide Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. there are three main steps. Iron Iii Chloride + Sodium Hydroxide.
From www.alamy.com
Iron (III) hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution Iron Iii Chloride + Sodium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a precipitation reaction between iron (iii) chloride and sodium. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles. Iron Iii Chloride + Sodium Hydroxide.
From www.alamy.com
Iron (III) chloride (FeCl3) solution. When sodium hydroxide is added to an iron (III) chloride Iron Iii Chloride + Sodium Hydroxide there are three main steps for writing the net ionic equation for fecl3 +. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. a precipitation reaction between iron (iii) chloride and. Iron Iii Chloride + Sodium Hydroxide.
From www.numerade.com
SOLVED Aqueous solutions of sodium hydroxide and iron(II) chloride are mixed Show two formula Iron Iii Chloride + Sodium Hydroxide a precipitation reaction between iron (iii) chloride and sodium. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. to balance a chemical equation, enter an equation of a chemical. Iron Iii Chloride + Sodium Hydroxide.
From www.youtube.com
NaOH+FeCl3=Fe(OH)3+NaCl Balanced EquationSodium hydroxide+Iron(iii)Chloride Balanced Equation Iron Iii Chloride + Sodium Hydroxide a precipitation reaction between iron (iii) chloride and sodium. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). one mole of ferric chloride [fecl 3], three moles of sodium. Iron Iii Chloride + Sodium Hydroxide.
From exombjkja.blob.core.windows.net
Iron 3 Chloride Ligands at Linda Chase blog Iron Iii Chloride + Sodium Hydroxide one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. there are three main steps for writing the net ionic equation for fecl3 +. a precipitation reaction between iron (iii) chloride and sodium. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq). Iron Iii Chloride + Sodium Hydroxide.
From stock.adobe.com
Chemical reactionPipette 0.25 M solution of iron(III) chloride (FeCl3) into 0.5 M solution of Iron Iii Chloride + Sodium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. a precipitation reaction between iron (iii) chloride and sodium. there are three main steps for writing the net ionic equation for fecl3 +. . Iron Iii Chloride + Sodium Hydroxide.
From www.youtube.com
iron III chloride + sodium hydroxide YouTube Iron Iii Chloride + Sodium Hydroxide a precipitation reaction between iron (iii) chloride and sodium. there are three main steps for writing the net ionic equation for fecl3 +. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles. Iron Iii Chloride + Sodium Hydroxide.
From www.slideserve.com
PPT Preview PowerPoint Presentation, free download ID6531641 Iron Iii Chloride + Sodium Hydroxide Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. there are three main steps for writing the net ionic equation for fecl3 +. a precipitation reaction between iron (iii) chloride and sodium. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a solution of. Iron Iii Chloride + Sodium Hydroxide.
From www.sciencephoto.com
Iron (III) chloride solution Stock Image C029/5914 Science Photo Library Iron Iii Chloride + Sodium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. a precipitation reaction between iron (iii) chloride and sodium. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). The balanced chemical equation for the reaction between iron (iii) chloride and. Iron Iii Chloride + Sodium Hydroxide.
From www.youtube.com
H9_Iron (III) chloride + Sodium hydroxide YouTube Iron Iii Chloride + Sodium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. there are three main steps for writing the net ionic equation for fecl3 +. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. The balanced chemical equation for the reaction between iron (iii) chloride. Iron Iii Chloride + Sodium Hydroxide.
From www.chegg.com
Solved Report Sheet Iron(III) Chloride + Sodium Hydroxide Iron Iii Chloride + Sodium Hydroxide a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. . Iron Iii Chloride + Sodium Hydroxide.
From www.slideserve.com
PPT Oral assessment PowerPoint Presentation, free download ID3444733 Iron Iii Chloride + Sodium Hydroxide a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. to balance a chemical equation, enter an equation of a chemical reaction and press the balance. Iron Iii Chloride + Sodium Hydroxide.
From www.slideserve.com
PPT Sodium hydroxide and hydrobromic acid PowerPoint Presentation, free download ID1328592 Iron Iii Chloride + Sodium Hydroxide there are three main steps for writing the net ionic equation for fecl3 +. a precipitation reaction between iron (iii) chloride and sodium. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. The. Iron Iii Chloride + Sodium Hydroxide.
From www.researchgate.net
What is the chemical difference between these iron III chloride solutions? ResearchGate Iron Iii Chloride + Sodium Hydroxide The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. a precipitation reaction between iron (iii). Iron Iii Chloride + Sodium Hydroxide.
From www.slideserve.com
PPT Double Displacement Complete and Ionic Equations PowerPoint Presentation ID2587470 Iron Iii Chloride + Sodium Hydroxide The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. a solution of iron(iii)chloride is mixed with a solution of. Iron Iii Chloride + Sodium Hydroxide.
From www.slideserve.com
PPT How are salts made and named? PowerPoint Presentation ID5348558 Iron Iii Chloride + Sodium Hydroxide a precipitation reaction between iron (iii) chloride and sodium. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles. Iron Iii Chloride + Sodium Hydroxide.
From www.youtube.com
How to Write the Formula for Iron (III) hydroxide YouTube Iron Iii Chloride + Sodium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). a precipitation reaction between iron (iii). Iron Iii Chloride + Sodium Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Chloride + Sodium Hydroxide a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. to balance a chemical equation, enter an equation of a chemical reaction and press the balance. Iron Iii Chloride + Sodium Hydroxide.
From www.sciencephoto.com
Iron (III) hydroxide precipitate Stock Image C027/9448 Science Photo Library Iron Iii Chloride + Sodium Hydroxide Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a precipitation reaction between iron (iii) chloride and sodium. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen. Iron Iii Chloride + Sodium Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Chloride + Sodium Hydroxide The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a precipitation reaction between iron (iii) chloride and sodium. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and. Iron Iii Chloride + Sodium Hydroxide.
From www.alamy.com
Iron (III) hydroxide precipitation. Test tube containing sodium hydroxide solution reacting with Iron Iii Chloride + Sodium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. there are three main steps for writing the net ionic equation for fecl3 +. a precipitation reaction between iron (iii) chloride and sodium. one. Iron Iii Chloride + Sodium Hydroxide.
From www.youtube.com
Reaction of Iron (III) chloride (FeCl3) with Ammonium hydroxide (NH4OH) YouTube Iron Iii Chloride + Sodium Hydroxide The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. there are three main steps for writing the net ionic equation for fecl3 +. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and. Iron Iii Chloride + Sodium Hydroxide.
From www.youtube.com
Addition sodium hydroxide to iron (II) chloride and iron (III) chloride MVI 6473 YouTube Iron Iii Chloride + Sodium Hydroxide there are three main steps for writing the net ionic equation for fecl3 +. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. a precipitation reaction between iron (iii) chloride and sodium. to balance a chemical equation, enter an equation of a chemical. Iron Iii Chloride + Sodium Hydroxide.
From pixels.com
Sodium Hydroxide And Iron IIi Chloride Photograph by Martyn F. Chillmaid/science Photo Library Iron Iii Chloride + Sodium Hydroxide a precipitation reaction between iron (iii) chloride and sodium. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). there are three main steps for writing the net ionic equation for fecl3. Iron Iii Chloride + Sodium Hydroxide.
From www.toppr.com
Iron III Hydroxide Formula Definition, Concepts and Examples Iron Iii Chloride + Sodium Hydroxide Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. a precipitation reaction between iron (iii) chloride and sodium. one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. there are three main steps for writing the net ionic. Iron Iii Chloride + Sodium Hydroxide.
From sochemvn.com
Iron (III) Chloride Solution FeCl3 38 HÓA CHẤT CƠ BẢN MIỀN NAM Iron Iii Chloride + Sodium Hydroxide one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. there are three main steps for writing the net ionic equation for fecl3 +. a solution of iron(iii)chloride is mixed. Iron Iii Chloride + Sodium Hydroxide.
From www.numerade.com
SOLVED In water, iron(III) chloride reacts with sodium hydroxide, producing solid iron(III Iron Iii Chloride + Sodium Hydroxide there are three main steps for writing the net ionic equation for fecl3 +. The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). to balance a chemical equation, enter an equation of. Iron Iii Chloride + Sodium Hydroxide.
From www.chegg.com
Solved Reaction 1 iron (III) chloride + sodium hydroxide Iron Iii Chloride + Sodium Hydroxide a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). there are three main steps for writing the net ionic equation for fecl3 +. Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. The balanced chemical equation for the reaction between iron. Iron Iii Chloride + Sodium Hydroxide.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Hydroxide... Course Hero Iron Iii Chloride + Sodium Hydroxide Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. there are three main steps for writing the net ionic equation for fecl3 +. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. one mole of ferric chloride [fecl 3], three moles of. Iron Iii Chloride + Sodium Hydroxide.
From stock.adobe.com
Chemical reactionRusty red iron(III) hydroxide precipitate (Fe(OH)3) in test tube formed Iron Iii Chloride + Sodium Hydroxide one mole of ferric chloride [fecl 3], three moles of sodium hydroxide [naoh] and zero moles of hydrogen chloride [hcl] react to. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron. Iron Iii Chloride + Sodium Hydroxide.
From www.numerade.com
SOLVEDIron(III) chloride is produced by the reaction of iron and chlorine (Figure 4.2). (a) If Iron Iii Chloride + Sodium Hydroxide Fecl_3 (aq) + 3naoh (aq) rarr fe (oh)_3 (s)darr + 3nacl (aq) most hydroxides are insoluble. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). one mole of ferric chloride. Iron Iii Chloride + Sodium Hydroxide.
From www.sciencephoto.com
Iron (III) hydroxide precipitate Stock Image A500/0413 Science Photo Library Iron Iii Chloride + Sodium Hydroxide a solution of iron(iii)chloride is mixed with a solution of sodium hydroxide and reacts to yield solid iron (iii). The balanced chemical equation for the reaction between iron (iii) chloride and sodium hydroxide in. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. there are three main steps for. Iron Iii Chloride + Sodium Hydroxide.