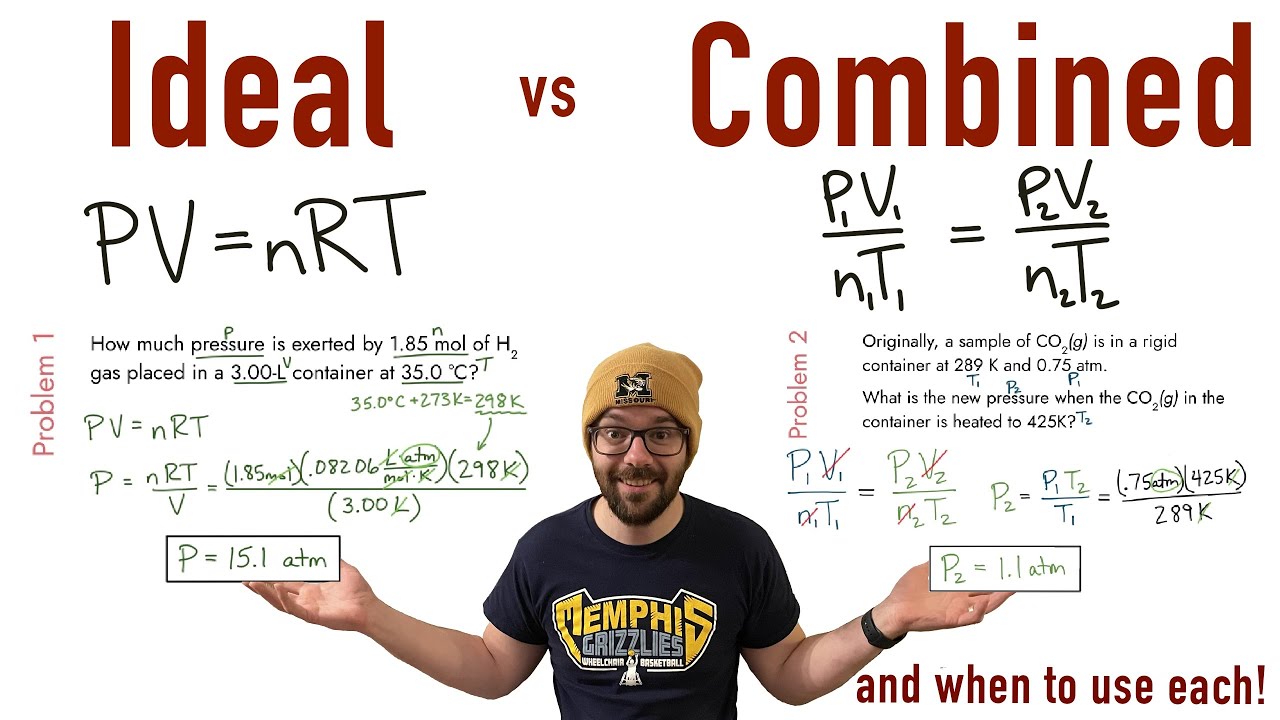
Combined Gas Law Intro Ws . 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. Use the combined gas law to solve the following problems: Use the combined gas law to solve the following problems: You have a gas at 25 c confined to a cylinder with a movable piston. What would the volume of the gas be at. Atoms & elements 4h 15m. V1p1t2 = v2p2t1 v1t2 = v2t. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. Intro to general chemistry 3h 51m. If i initially have a gas at a pressure of 12. Which of the following actions would double the gas pressure? When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. A gas sample contained in a cylinder equipped with a. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr.
from www.youtube.com
What would the volume of the gas be at. Use the combined gas law to solve the following problems: A gas sample contained in a cylinder equipped with a. You have a gas at 25 c confined to a cylinder with a movable piston. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. Atoms & elements 4h 15m. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. Which of the following actions would double the gas pressure? When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. If i initially have a gas at a pressure of 12.
Ideal and Combined Gas Laws + When to use them! (AP Chemistry) YouTube
Combined Gas Law Intro Ws Intro to general chemistry 3h 51m. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. Intro to general chemistry 3h 51m. V1p1t2 = v2p2t1 v1t2 = v2t. If i initially have a gas at a pressure of 12. What would the volume of the gas be at. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. A gas sample contained in a cylinder equipped with a. Which of the following actions would double the gas pressure? Use the combined gas law to solve the following problems: Atoms & elements 4h 15m. Use the combined gas law to solve the following problems: You have a gas at 25 c confined to a cylinder with a movable piston.
From jacksofscience.com
The Combined Gas Law Jacks Of Science Combined Gas Law Intro Ws Atoms & elements 4h 15m. If i initially have a gas at a pressure of 12. Intro to general chemistry 3h 51m. A gas sample contained in a cylinder equipped with a. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4.. Combined Gas Law Intro Ws.
From www.sciencephoto.com
Combined gas law, artwork Stock Image C013/4731 Science Photo Library Combined Gas Law Intro Ws Which of the following actions would double the gas pressure? Atoms & elements 4h 15m. What would the volume of the gas be at. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. Intro to general chemistry 3h 51m. If i initially have a gas at a pressure of 12.. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Combined Gas Law Intro Ws Atoms & elements 4h 15m. Use the combined gas law to solve the following problems: A gas sample contained in a cylinder equipped with a. Use the combined gas law to solve the following problems: 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the. Combined Gas Law Intro Ws.
From davida.davivienda.com
Combined Gas Law Worksheet Answers Printable Word Searches Combined Gas Law Intro Ws Use the combined gas law to solve the following problems: If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. Which of the following actions would double the gas pressure? 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume. Combined Gas Law Intro Ws.
From studylib.net
The Combined Gas Law Combined Gas Law Intro Ws When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. What would the volume of the gas be at. If i initially have a gas at a pressure of 12. Intro to general chemistry 3h 51m. Use the combined gas law to solve the following. Combined Gas Law Intro Ws.
From www.shutterstock.com
Gas Laws Infographic Diagram Showing Combined vetor stock (livre de Combined Gas Law Intro Ws If i initially have a gas at a pressure of 12. You have a gas at 25 c confined to a cylinder with a movable piston. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. What would the volume of the gas be at. A gas sample contained in a. Combined Gas Law Intro Ws.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Combined Gas Law Intro Ws What would the volume of the gas be at. Atoms & elements 4h 15m. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0. Combined Gas Law Intro Ws.
From www.youtube.com
Combined Gas Law YouTube Combined Gas Law Intro Ws If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. V1p1t2 = v2p2t1 v1t2 = v2t. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. Atoms & elements 4h 15m. 1) if i initially have 4.0 l of a gas. Combined Gas Law Intro Ws.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Combined Gas Law Intro Ws When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. Use the combined gas law to solve the following problems: V1p1t2 = v2p2t1 v1t2 = v2t. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the. Combined Gas Law Intro Ws.
From www.youtube.com
Ideal and Combined Gas Laws + When to use them! (AP Chemistry) YouTube Combined Gas Law Intro Ws When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. Which of the following actions would double the gas pressure? Atoms & elements 4h 15m.. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID818392 Combined Gas Law Intro Ws Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. You have a gas at 25 c confined to a cylinder with a movable piston. Which of the following actions would double the gas pressure? Atoms & elements 4h 15m. If i initially have a gas at a pressure of 12. Combined Gas Law Intro Ws.
From owlcation.com
The Theories and Behavior of Gas Owlcation Combined Gas Law Intro Ws Atoms & elements 4h 15m. Use the combined gas law to solve the following problems: Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. If i initially have a gas at a pressure of 12. When you use the combined gas law paired with dalton's law, remember that a gas. Combined Gas Law Intro Ws.
From www.studypool.com
SOLUTION The combined gas law science Studypool Combined Gas Law Intro Ws Use the combined gas law to solve the following problems: If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. You have a gas at 25 c confined to a cylinder with a movable piston. Atoms & elements 4h 15m. V1p1t2 = v2p2t1 v1t2 = v2t. Which of the following. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT Gas Law Notes Chemistry Semester II PowerPoint Presentation, free Combined Gas Law Intro Ws Atoms & elements 4h 15m. You have a gas at 25 c confined to a cylinder with a movable piston. If i initially have a gas at a pressure of 12. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. A gas sample contained. Combined Gas Law Intro Ws.
From www.youtube.com
Lec8 The Combined Gas Law YouTube Combined Gas Law Intro Ws Intro to general chemistry 3h 51m. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. What would the volume of the gas be at. Atoms & elements 4h 15m. A gas sample contained in a cylinder equipped with a. Which of. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Combined Gas Law Intro Ws 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. V1p1t2 = v2p2t1 v1t2 = v2t. If i initially have a gas at a pressure of 12. Use the combined gas law to solve the following problems: Intro to general chemistry 3h. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT Combined Gas Law Avogadro’s Principle PowerPoint Presentation Combined Gas Law Intro Ws Use the combined gas law to solve the following problems: When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. If i initially have a gas at a pressure of 12. Combined gas law 1 a gas has a volume of 800.0 ml at minus. Combined Gas Law Intro Ws.
From slidesharetrick.blogspot.com
The Combined Gas Law slidesharetrick Combined Gas Law Intro Ws Atoms & elements 4h 15m. If i initially have a gas at a pressure of 12. Intro to general chemistry 3h 51m. Use the combined gas law to solve the following problems: Which of the following actions would double the gas pressure? 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will. Combined Gas Law Intro Ws.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Combined Gas Law Intro Ws V1p1t2 = v2p2t1 v1t2 = v2t. Atoms & elements 4h 15m. Which of the following actions would double the gas pressure? Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature.. Combined Gas Law Intro Ws.
From www.studypool.com
SOLUTION Gay lussacs gas law combined gas law formula Studypool Combined Gas Law Intro Ws Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. What would the volume of the gas be at. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. V1p1t2 = v2p2t1 v1t2. Combined Gas Law Intro Ws.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Combined Gas Law Intro Ws V1p1t2 = v2p2t1 v1t2 = v2t. If i initially have a gas at a pressure of 12. Use the combined gas law to solve the following problems: What would the volume of the gas be at. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. Intro to general chemistry. Combined Gas Law Intro Ws.
From slideplayer.com
Combined Gas Law Equation Problems ppt download Combined Gas Law Intro Ws Intro to general chemistry 3h 51m. A gas sample contained in a cylinder equipped with a. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. V1p1t2 = v2p2t1 v1t2 = v2t. What would the volume of the gas be at. You have a gas. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378 Combined Gas Law Intro Ws 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. A gas sample contained in a cylinder equipped with a. V1p1t2 = v2p2t1 v1t2 = v2t. If i initially have a gas at a pressure of 12 atm, a volume of 23. Combined Gas Law Intro Ws.
From esaaderiksen.blogspot.com
20+ Combined Gas Law Calculator EsaadEriksen Combined Gas Law Intro Ws If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. When you use the combined gas law paired with dalton's law, remember. Combined Gas Law Intro Ws.
From www.showme.com
ShowMe derivation of combined gas law Combined Gas Law Intro Ws Atoms & elements 4h 15m. Use the combined gas law to solve the following problems: When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. What would the volume of the gas be at. A gas sample contained in a cylinder equipped with a. Which. Combined Gas Law Intro Ws.
From studylib.net
The Combined Gas Law Combined Gas Law Intro Ws V1p1t2 = v2p2t1 v1t2 = v2t. Atoms & elements 4h 15m. Use the combined gas law to solve the following problems: You have a gas at 25 c confined to a cylinder with a movable piston. What would the volume of the gas be at. A gas sample contained in a cylinder equipped with a. If i initially have a. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT The Combined and Ideal Gas Laws PowerPoint Presentation, free Combined Gas Law Intro Ws Which of the following actions would double the gas pressure? Intro to general chemistry 3h 51m. V1p1t2 = v2p2t1 v1t2 = v2t. You have a gas at 25 c confined to a cylinder with a movable piston. If i initially have a gas at a pressure of 12. Use the combined gas law to solve the following problems: Atoms &. Combined Gas Law Intro Ws.
From learningnevestamyq.z21.web.core.windows.net
Combined Gas Law Problems With Answers Pdf Combined Gas Law Intro Ws Use the combined gas law to solve the following problems: A gas sample contained in a cylinder equipped with a. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. Atoms & elements 4h 15m. Which of the following actions would double the gas pressure?. Combined Gas Law Intro Ws.
From www.youtube.com
13.1 Combined Gas Law YouTube Combined Gas Law Intro Ws If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. A gas sample contained in a cylinder equipped with a. Use the combined gas law to solve the following problems: You have a gas at 25 c confined to a cylinder with a movable piston. Intro to general chemistry 3h. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT The Gas Laws PowerPoint Presentation, free download ID3358137 Combined Gas Law Intro Ws If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. V1p1t2 = v2p2t1 v1t2 = v2t. Which of the following actions would double the gas. Combined Gas Law Intro Ws.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4199554 Combined Gas Law Intro Ws Intro to general chemistry 3h 51m. You have a gas at 25 c confined to a cylinder with a movable piston. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. Use the combined gas law to solve the following problems: Combined gas law 1. Combined Gas Law Intro Ws.
From www.studypool.com
SOLUTION 8 1 the combined gas law Studypool Combined Gas Law Intro Ws Use the combined gas law to solve the following problems: When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with. A gas sample contained in a cylinder equipped with a. If i initially have a gas at a pressure of 12 atm, a volume of. Combined Gas Law Intro Ws.
From www.storyboardthat.com
Combined Gas Law Storyboard by f0a2bdd1 Combined Gas Law Intro Ws Atoms & elements 4h 15m. You have a gas at 25 c confined to a cylinder with a movable piston. What would the volume of the gas be at. Intro to general chemistry 3h 51m. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated with.. Combined Gas Law Intro Ws.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Combined Gas Law Intro Ws If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature. Use the combined gas law to solve the following problems: V1p1t2 = v2p2t1 v1t2 = v2t. When you use the combined gas law paired with dalton's law, remember that a gas collected over water is always considered to be saturated. Combined Gas Law Intro Ws.
From www.sliderbase.com
Gas Laws Presentation Chemistry Combined Gas Law Intro Ws 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. Combined gas law 1 a gas has a volume of 800.0 ml at minus 23.00 oc and 300.0 torr. Which of the following actions would double the gas pressure? When you use. Combined Gas Law Intro Ws.