Non Human Errors In Titration Labs . For example, if a sample solution has been left open, a small amount of the solution may have evaporated. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Human error can also pose many limitations to a titration experiment. First, there is an intrinsic error of the. There are several types of errors that can make titration result differ from the reality. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. To avoid errors, use clean equipment, keep notes. Have you ever wondered why your titration results are not reproducible? This blog post discusses the most common random and systematic errors that can happen during a.
from www.studocu.com
First, there is an intrinsic error of the. To avoid errors, use clean equipment, keep notes. There are several types of errors that can make titration result differ from the reality. This blog post discusses the most common random and systematic errors that can happen during a. Human error can also pose many limitations to a titration experiment. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. Have you ever wondered why your titration results are not reproducible? For example, if a sample solution has been left open, a small amount of the solution may have evaporated. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer.
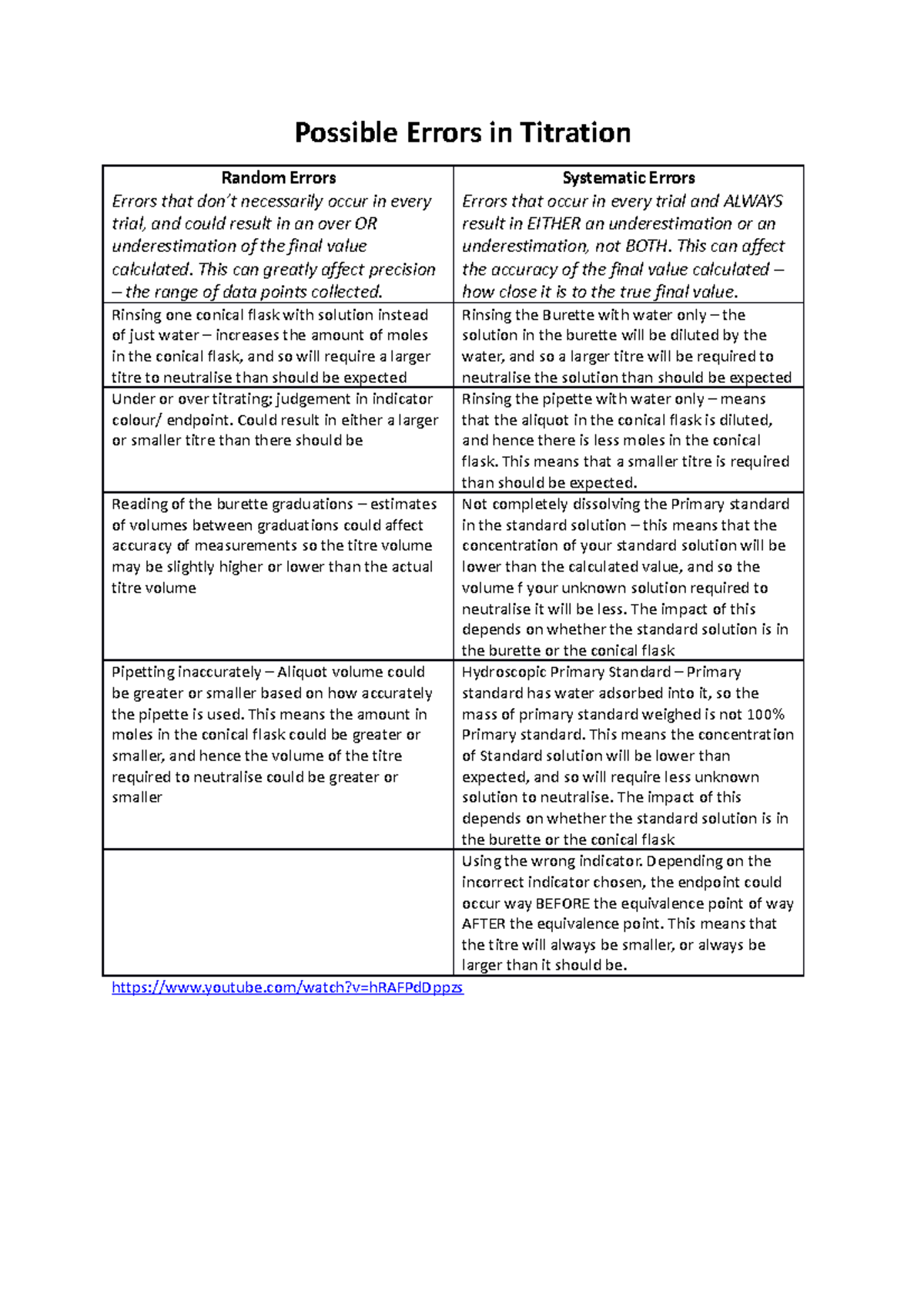
Random and Systematic Errors in Titration Possible Errors in
Non Human Errors In Titration Labs One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Human error can also pose many limitations to a titration experiment. For example, if a sample solution has been left open, a small amount of the solution may have evaporated. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. There are several types of errors that can make titration result differ from the reality. This blog post discusses the most common random and systematic errors that can happen during a. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Have you ever wondered why your titration results are not reproducible? First, there is an intrinsic error of the. To avoid errors, use clean equipment, keep notes.
From www.youtube.com
Errors in titrations YouTube Non Human Errors In Titration Labs Have you ever wondered why your titration results are not reproducible? One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Human error can also pose many limitations to a titration experiment. Titration is a common laboratory method in chemistry that is. Non Human Errors In Titration Labs.
From pdfslide.net
(PDF) Automatic Titration Lab Equip Titration increases accuracy Non Human Errors In Titration Labs Human error can also pose many limitations to a titration experiment. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. There are several types of errors that can make titration result differ from the reality. This blog post discusses the most common random and systematic errors that can. Non Human Errors In Titration Labs.
From kieranhobbs.blogspot.com
Precautions During Titration / Lab S Safety Precautions Redox Titration Non Human Errors In Titration Labs There are several types of errors that can make titration result differ from the reality. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in. Non Human Errors In Titration Labs.
From beyondlabz.freshdesk.com
Titrations Lab Overview Beyond Labz Non Human Errors In Titration Labs Have you ever wondered why your titration results are not reproducible? For example, if a sample solution has been left open, a small amount of the solution may have evaporated. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Human error. Non Human Errors In Titration Labs.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Non Human Errors In Titration Labs Human error can also pose many limitations to a titration experiment. For example, if a sample solution has been left open, a small amount of the solution may have evaporated. To avoid errors, use clean equipment, keep notes. There are several types of errors that can make titration result differ from the reality. First, there is an intrinsic error of. Non Human Errors In Titration Labs.
From www.degruyter.com
Human Errors in a Routine Analytical Laboratory—Classification Non Human Errors In Titration Labs Human error can also pose many limitations to a titration experiment. First, there is an intrinsic error of the. For example, if a sample solution has been left open, a small amount of the solution may have evaporated. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. This. Non Human Errors In Titration Labs.
From www.chegg.com
Solved Fit Page Height 8* Sources of Error in Titrations Non Human Errors In Titration Labs For example, if a sample solution has been left open, a small amount of the solution may have evaporated. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To avoid errors, use clean equipment, keep notes. Human error can also pose. Non Human Errors In Titration Labs.
From www.slideserve.com
PPT Non aqueous Titration theory and principles ppt PowerPoint Non Human Errors In Titration Labs There are several types of errors that can make titration result differ from the reality. Have you ever wondered why your titration results are not reproducible? Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. One example of a systematic error could be using a ph meter that. Non Human Errors In Titration Labs.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Non Human Errors In Titration Labs One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Human error can also pose many limitations to a titration experiment. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution.. Non Human Errors In Titration Labs.
From letitsnowglobe.co.uk
Titration procedure pdf Non Human Errors In Titration Labs First, there is an intrinsic error of the. There are several types of errors that can make titration result differ from the reality. This blog post discusses the most common random and systematic errors that can happen during a. Human error can also pose many limitations to a titration experiment. Have you ever wondered why your titration results are not. Non Human Errors In Titration Labs.
From mavink.com
Titration Reaction Non Human Errors In Titration Labs One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Human error can also pose many limitations to a titration experiment. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution.. Non Human Errors In Titration Labs.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Non Human Errors In Titration Labs Have you ever wondered why your titration results are not reproducible? Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. There are several types of errors that can make titration result differ from the reality. Human error can also pose many limitations to a titration experiment. One example. Non Human Errors In Titration Labs.
From itnewstoday.net
Fixed How To Fix Experimental Error Sources In Titration. IT News Today Non Human Errors In Titration Labs One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Human error can also pose many limitations to a titration experiment. First, there is an intrinsic error of the. Titration is a common laboratory method in chemistry that is used to determine. Non Human Errors In Titration Labs.
From mmerevise.co.uk
Titrations and Uncertainties MME Non Human Errors In Titration Labs Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. Human error can also pose many limitations to a titration experiment. This blog post discusses the most common random and systematic errors that can happen during a. To avoid errors, use clean equipment, keep notes. For example, if a. Non Human Errors In Titration Labs.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Non Human Errors In Titration Labs For example, if a sample solution has been left open, a small amount of the solution may have evaporated. First, there is an intrinsic error of the. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. To avoid errors, use clean equipment, keep notes. This blog post discusses. Non Human Errors In Titration Labs.
From www.scribd.com
Titration (Chemistry Experiment Report) PDF Titration Chemistry Non Human Errors In Titration Labs This blog post discusses the most common random and systematic errors that can happen during a. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. First, there is an intrinsic error of the. Titration is a common laboratory method in chemistry. Non Human Errors In Titration Labs.
From www.slideserve.com
PPT Volumetric Analysis (Titration) PowerPoint Presentation, free Non Human Errors In Titration Labs For example, if a sample solution has been left open, a small amount of the solution may have evaporated. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads. Non Human Errors In Titration Labs.
From www.slideserve.com
PPT Measurements and Sources of Errors PowerPoint Presentation, free Non Human Errors In Titration Labs This blog post discusses the most common random and systematic errors that can happen during a. Human error can also pose many limitations to a titration experiment. To avoid errors, use clean equipment, keep notes. Have you ever wondered why your titration results are not reproducible? Titration is a common laboratory method in chemistry that is used to determine the. Non Human Errors In Titration Labs.
From www.youtube.com
Random and Systematic Errors in Titrations YouTube Non Human Errors In Titration Labs This blog post discusses the most common random and systematic errors that can happen during a. For example, if a sample solution has been left open, a small amount of the solution may have evaporated. To avoid errors, use clean equipment, keep notes. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so. Non Human Errors In Titration Labs.
From www.answersarena.com
[Solved] Review the list of common titration errors. Dete Non Human Errors In Titration Labs Human error can also pose many limitations to a titration experiment. For example, if a sample solution has been left open, a small amount of the solution may have evaporated. This blog post discusses the most common random and systematic errors that can happen during a. To avoid errors, use clean equipment, keep notes. There are several types of errors. Non Human Errors In Titration Labs.
From medsolut.com
Titration Design of the experiment MedSolut Non Human Errors In Titration Labs There are several types of errors that can make titration result differ from the reality. First, there is an intrinsic error of the. This blog post discusses the most common random and systematic errors that can happen during a. To avoid errors, use clean equipment, keep notes. One example of a systematic error could be using a ph meter that. Non Human Errors In Titration Labs.
From www.expii.com
Types of Error — Overview & Comparison Expii Non Human Errors In Titration Labs For example, if a sample solution has been left open, a small amount of the solution may have evaporated. This blog post discusses the most common random and systematic errors that can happen during a. Have you ever wondered why your titration results are not reproducible? Human error can also pose many limitations to a titration experiment. There are several. Non Human Errors In Titration Labs.
From mungfali.com
Acid Base Titration Experiment Non Human Errors In Titration Labs To avoid errors, use clean equipment, keep notes. There are several types of errors that can make titration result differ from the reality. For example, if a sample solution has been left open, a small amount of the solution may have evaporated. This blog post discusses the most common random and systematic errors that can happen during a. Have you. Non Human Errors In Titration Labs.
From scite.ai
Titration errors in chelometric titrations employing ionselective Non Human Errors In Titration Labs There are several types of errors that can make titration result differ from the reality. This blog post discusses the most common random and systematic errors that can happen during a. Human error can also pose many limitations to a titration experiment. To avoid errors, use clean equipment, keep notes. Titration is a common laboratory method in chemistry that is. Non Human Errors In Titration Labs.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Non Human Errors In Titration Labs One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To avoid errors, use clean equipment, keep notes. Have you ever wondered why your titration results are not reproducible? For example, if a sample solution has been left open, a small amount. Non Human Errors In Titration Labs.
From www.studocu.com
Random and Systematic Errors in Titration Possible Errors in Non Human Errors In Titration Labs For example, if a sample solution has been left open, a small amount of the solution may have evaporated. This blog post discusses the most common random and systematic errors that can happen during a. First, there is an intrinsic error of the. To avoid errors, use clean equipment, keep notes. One example of a systematic error could be using. Non Human Errors In Titration Labs.
From www.youtube.com
Sources of errors in Medical Laboratory PreAnalytical Errors Non Human Errors In Titration Labs To avoid errors, use clean equipment, keep notes. Have you ever wondered why your titration results are not reproducible? One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. For example, if a sample solution has been left open, a small amount. Non Human Errors In Titration Labs.
From www.slideserve.com
PPT Scientific Method PowerPoint Presentation, free download ID4180522 Non Human Errors In Titration Labs Have you ever wondered why your titration results are not reproducible? For example, if a sample solution has been left open, a small amount of the solution may have evaporated. First, there is an intrinsic error of the. There are several types of errors that can make titration result differ from the reality. Human error can also pose many limitations. Non Human Errors In Titration Labs.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Non Human Errors In Titration Labs For example, if a sample solution has been left open, a small amount of the solution may have evaporated. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. Have you ever wondered why your titration results are not reproducible? This blog post discusses the most common random and. Non Human Errors In Titration Labs.
From beingchemist.com
Titration Being Chemist Non Human Errors In Titration Labs Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. To avoid errors, use clean equipment, keep notes. Have you ever wondered why your titration results are not reproducible? For example, if a sample solution has been left open, a small amount of the solution may have evaporated. Human. Non Human Errors In Titration Labs.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Non Human Errors In Titration Labs To avoid errors, use clean equipment, keep notes. For example, if a sample solution has been left open, a small amount of the solution may have evaporated. There are several types of errors that can make titration result differ from the reality. First, there is an intrinsic error of the. This blog post discusses the most common random and systematic. Non Human Errors In Titration Labs.
From mavink.com
4 Types Of Titration Curves Non Human Errors In Titration Labs Human error can also pose many limitations to a titration experiment. There are several types of errors that can make titration result differ from the reality. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. One example of a systematic error could be using a ph meter that. Non Human Errors In Titration Labs.
From www.youtube.com
How to calculate uncertainty in titration YouTube Non Human Errors In Titration Labs First, there is an intrinsic error of the. To avoid errors, use clean equipment, keep notes. This blog post discusses the most common random and systematic errors that can happen during a. There are several types of errors that can make titration result differ from the reality. One example of a systematic error could be using a ph meter that. Non Human Errors In Titration Labs.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Non Human Errors In Titration Labs Human error can also pose many limitations to a titration experiment. This blog post discusses the most common random and systematic errors that can happen during a. Titration is a common laboratory method in chemistry that is used to determine the concentration of a substance in a solution. Have you ever wondered why your titration results are not reproducible? One. Non Human Errors In Titration Labs.
From go.labmanager.com
Lab Manager Impact of Human Error in Titration inar Non Human Errors In Titration Labs This blog post discusses the most common random and systematic errors that can happen during a. Have you ever wondered why your titration results are not reproducible? Human error can also pose many limitations to a titration experiment. To avoid errors, use clean equipment, keep notes. For example, if a sample solution has been left open, a small amount of. Non Human Errors In Titration Labs.