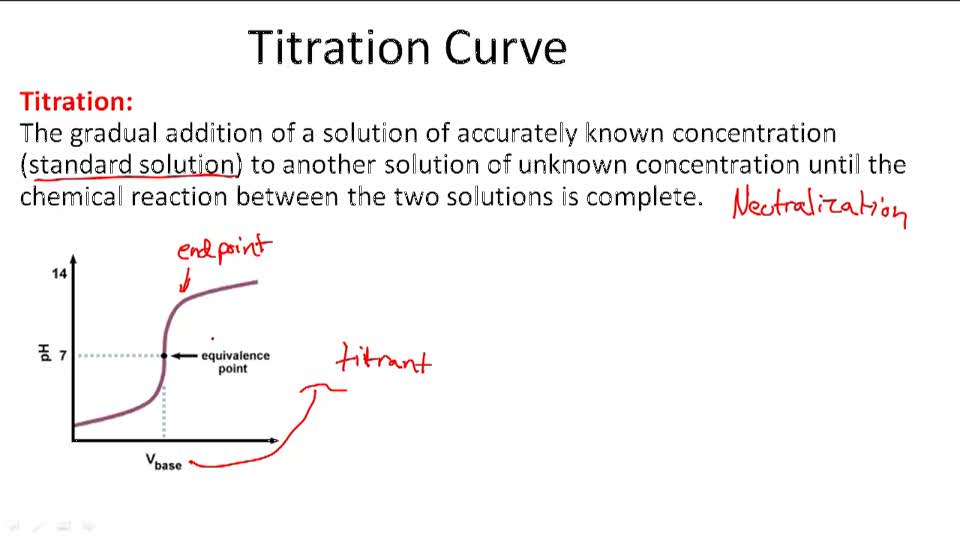
Back Titration Problems . Calculate analyte concentrations given experimental data from a back titration. Then using mole ratio from reaction 2, we can. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. From the diagram, we can start with the titration first and determine the amount of naoh used. The resulting mixture is then. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Back titrations are used when: A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess.
from mavink.com
Calculate analyte concentrations given experimental data from a back titration. The resulting mixture is then. Then using mole ratio from reaction 2, we can. Back titrations are used when: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. From the diagram, we can start with the titration first and determine the amount of naoh used. Reactant a of unknown concentration is reacted with excess. You are asked to determine the mass of calcium.
Back Titration Calculations
Back Titration Problems Then using mole ratio from reaction 2, we can. Then using mole ratio from reaction 2, we can. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Calculate analyte concentrations given experimental data from a back titration. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess. The resulting mixture is then. From the diagram, we can start with the titration first and determine the amount of naoh used. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Back titrations are used when:
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Back Titration Problems From the diagram, we can start with the titration first and determine the amount of naoh used. Calculate analyte concentrations given experimental data from a back titration. You are asked to determine the mass of calcium. Then using mole ratio from reaction 2, we can. A back titration is a titration method where the concentration of an analyte is determined. Back Titration Problems.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Back Titration Problems The resulting mixture is then. Back titrations are used when: You are asked to determine the mass of calcium. Then using mole ratio from reaction 2, we can. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. A 1.0000 gram sample of k2co3 (138.2055. Back Titration Problems.
From www.facebook.com
10/18/24 Live with NORML You've got burning questions. We've got Back Titration Problems A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Reactant a of unknown concentration is reacted with excess. From the diagram, we can start with the titration first and determine the amount of naoh used. Then using mole ratio from reaction 2, we can.. Back Titration Problems.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Back Titration Problems Then using mole ratio from reaction 2, we can. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Calculate analyte concentrations given experimental data from a back titration. The resulting mixture is then. Back titration is used to find the number of moles of. Back Titration Problems.
From kit.co
Back Titration Problems With Answers Back Titration Problems From the diagram, we can start with the titration first and determine the amount of naoh used. Then using mole ratio from reaction 2, we can. Reactant a of unknown concentration is reacted with excess. The resulting mixture is then. You are asked to determine the mass of calcium. Calculate analyte concentrations given experimental data from a back titration. A. Back Titration Problems.
From www.facebook.com
10/18/24 Live with NORML You've got burning questions. We've got Back Titration Problems Back titrations are used when: Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. You are. Back Titration Problems.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Back Titration Problems From the diagram, we can start with the titration first and determine the amount of naoh used. Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Calculate analyte concentrations given experimental data from a back titration. You are asked to determine. Back Titration Problems.
From www.youtube.com
Back titration YouTube Back Titration Problems Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Calculate analyte concentrations given experimental data from a back titration. Back titrations are used when: From the diagram, we can start with the titration first and determine the amount of naoh used. Reactant a of. Back Titration Problems.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Back Titration Problems From the diagram, we can start with the titration first and determine the amount of naoh used. Then using mole ratio from reaction 2, we can. Back titrations are used when: Reactant a of unknown concentration is reacted with excess. The resulting mixture is then. You are asked to determine the mass of calcium. Back titration is used to find. Back Titration Problems.
From www.vrogue.co
What Is Back Titration Lexiebilclayton vrogue.co Back Titration Problems You are asked to determine the mass of calcium. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Reactant. Back Titration Problems.
From www.youtube.com
Back Titration Example YouTube Back Titration Problems Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. You are asked to determine the mass of calcium. From the diagram, we can start with the titration first and determine the amount of naoh used. A back titration is a titration method where the. Back Titration Problems.
From www.chegg.com
Solved The solution to the starred problem is in Appendix Back Titration Problems Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then. Then using mole ratio from reaction 2, we can. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Calculate analyte. Back Titration Problems.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Back Titration Problems Then using mole ratio from reaction 2, we can. Back titrations are used when: From the diagram, we can start with the titration first and determine the amount of naoh used. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. The resulting mixture is. Back Titration Problems.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Back Titration Problems Reactant a of unknown concentration is reacted with excess. The resulting mixture is then. Calculate analyte concentrations given experimental data from a back titration. Then using mole ratio from reaction 2, we can. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. You are asked to determine the mass of. Back Titration Problems.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Back Titration Problems A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. From the diagram, we can start with the titration first and determine the amount of naoh used. You are asked to determine the mass of calcium. A back titration is a titration method where the concentration of an analyte is determined. Back Titration Problems.
From www.youtube.com
Back Titrations Example YouTube Back Titration Problems A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. From the diagram, we can start with the titration first. Back Titration Problems.
From en.differbetween.com
Difference Between Back Titration and Direct Titration Differbetween Back Titration Problems The resulting mixture is then. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. Back titrations are used when: From the diagram, we can start. Back Titration Problems.
From studylib.net
Titration Practice Worksheet Back Titration Problems Calculate analyte concentrations given experimental data from a back titration. From the diagram, we can start with the titration first and determine the amount of naoh used. You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess. Then using mole ratio from reaction 2, we can. A 1.0000 gram sample of k2co3. Back Titration Problems.
From www.facebook.com
10/18/24 Live with NORML You've got burning questions. We've got Back Titration Problems Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. From the diagram, we can start with the titration first and determine the amount of naoh used. Calculate analyte concentrations given experimental data from a back titration. Then using mole ratio from reaction 2, we. Back Titration Problems.
From wealthlesslacas.weebly.com
Back Titration Problems And Solutions LINK Back Titration Problems Calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Then using mole ratio from reaction 2, we can. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant. Back Titration Problems.
From mungfali.com
Titration Example Problem Back Titration Problems Back titrations are used when: A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. From the diagram, we can start with the titration first and. Back Titration Problems.
From gioltgugv.blob.core.windows.net
Titration Lab Problems at Dorothy Stephenson blog Back Titration Problems You are asked to determine the mass of calcium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Back titrations are used when: From the diagram, we can start with the titration first and determine the amount of naoh used. Back titration is used to find the number of moles. Back Titration Problems.
From mavink.com
Titration Reaction Back Titration Problems Calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. From the diagram, we can start with the titration first and determine the amount of naoh used. Back titration is used to find the number of moles of a substance by. Back Titration Problems.
From facts.net
13 Surprising Facts About Back Titration Back Titration Problems Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. The resulting mixture is then. Back titrations are used when: Calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make. Back Titration Problems.
From mavink.com
Back Titration Calculations Back Titration Problems The resulting mixture is then. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Then using mole ratio from reaction 2, we can. Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. A back. Back Titration Problems.
From hxeukuiaa.blob.core.windows.net
Titration Sample Problems With Answers at Vanessa Farrar blog Back Titration Problems Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Then using mole ratio from reaction 2, we can. Back titrations are used when: Back titration is used to find the number of moles of a substance by reacting it with an. Back Titration Problems.
From www.youtube.com
Back Titrations YouTube Back Titration Problems Back titrations are used when: Calculate analyte concentrations given experimental data from a back titration. You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess. Then using mole ratio from reaction 2, we can. From the diagram, we can start with the titration first and determine the amount of naoh used. A. Back Titration Problems.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Back Titration Problems From the diagram, we can start with the titration first and determine the amount of naoh used. Calculate analyte concentrations given experimental data from a back titration. Back titrations are used when: Back titration is used to find the number of moles of a substance by reacting it with an excess volume of reactant of known concentration. Reactant a of. Back Titration Problems.