Amino Acids Zwitterion In Water . Because they carry both positive and. Zwitterions in simple amino acid solutions. In water, the ionic attractions. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. They act as buffer solutions as they resist any changes in ph when small. But, unlike simple amphoteric compounds that may only form. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. This again reflects the presence of the zwitterions. An amino acid has both a basic amine group and an acidic carboxylic acid group.
from www.alamy.com
When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. An amino acid has both a basic amine group and an acidic carboxylic acid group. Zwitterions in simple amino acid solutions. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. But, unlike simple amphoteric compounds that may only form. This again reflects the presence of the zwitterions. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. In water, the ionic attractions. They act as buffer solutions as they resist any changes in ph when small.
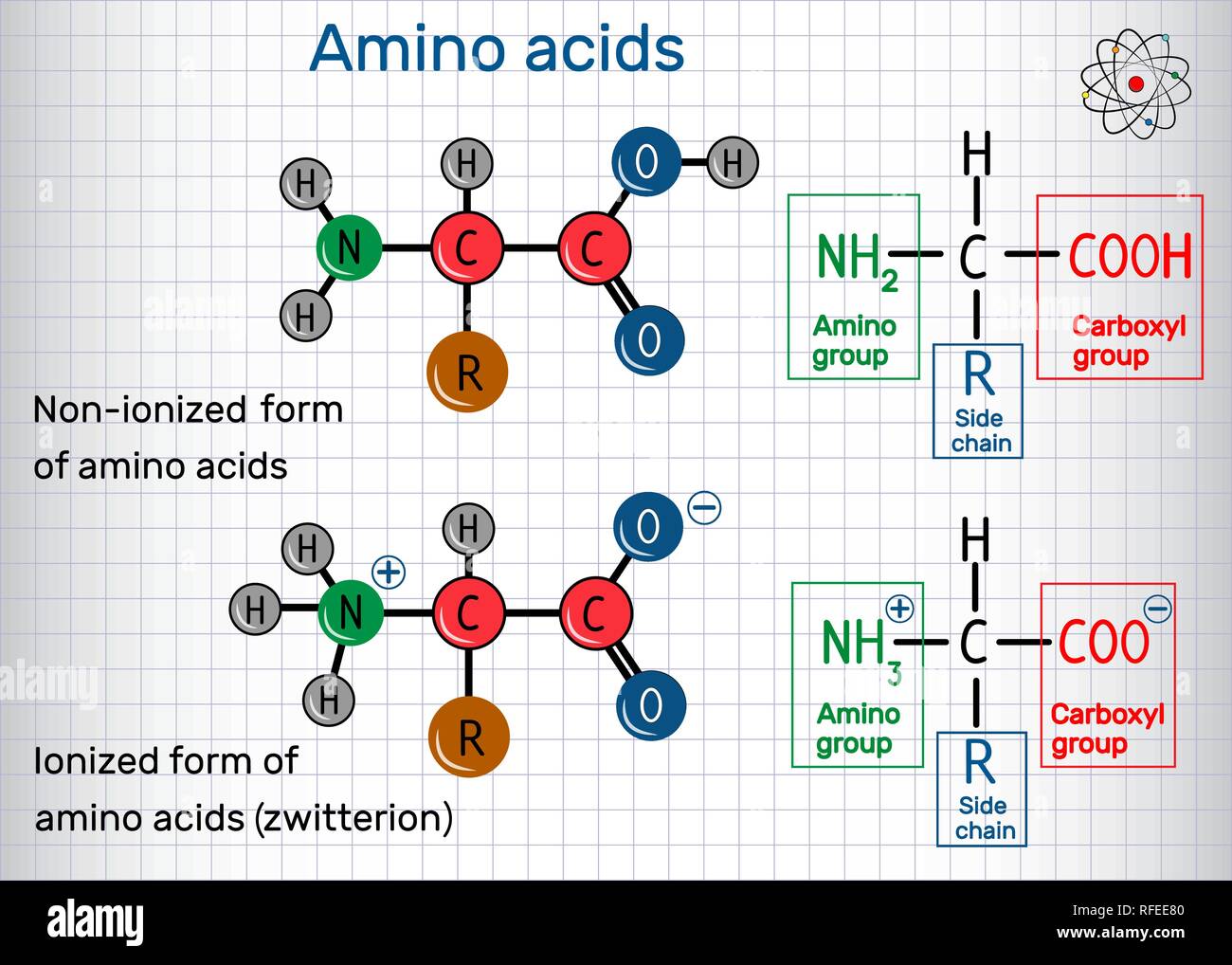
General formula of amino acids, ionized and nonionized (zwitterion
Amino Acids Zwitterion In Water Because they carry both positive and. Because they carry both positive and. But, unlike simple amphoteric compounds that may only form. They act as buffer solutions as they resist any changes in ph when small. Zwitterions in simple amino acid solutions. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. This again reflects the presence of the zwitterions. In water, the ionic attractions. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. An amino acid has both a basic amine group and an acidic carboxylic acid group.
From www.alamy.com
General formula of amino acids, ionized and nonionized (zwitterion Amino Acids Zwitterion In Water But, unlike simple amphoteric compounds that may only form. Zwitterions in simple amino acid solutions. In water, the ionic attractions. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. The zwitterionic state of amino acids plays various. Amino Acids Zwitterion In Water.
From www.numerade.com
SOLVED 1. Glucose is the sugar whose common name is dextrose or grape Amino Acids Zwitterion In Water In water, the ionic attractions. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. An amino acid has both a basic amine group and an acidic carboxylic acid group. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. A solution of amino acids in water will exist as zwitterions with. Amino Acids Zwitterion In Water.
From microbiologynotes.org
Amino acids physical, chemical properties and peptide bond Amino Acids Zwitterion In Water The zwitterion of an amino acid exists at a ph equal to the isoelectric point. This again reflects the presence of the zwitterions. An amino acid has both a basic amine group and an acidic carboxylic acid group. They act as buffer solutions as they resist any changes in ph when small. A solution of amino acids in water will. Amino Acids Zwitterion In Water.
From www.chegg.com
Solved The following amino acids are drawn in zwitterion Amino Acids Zwitterion In Water This again reflects the presence of the zwitterions. Because they carry both positive and. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Zwitterions in simple amino acid solutions. An amino acid has both a basic amine group and an acidic carboxylic acid. Amino Acids Zwitterion In Water.
From www.youtube.com
physical properties of amino acids(ampholytes,zwitterion,isoelectric ph Amino Acids Zwitterion In Water When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. A solution of amino acids in. Amino Acids Zwitterion In Water.
From www.lecturio.com
Amino Acids & Proteins Chemistry for Physicians Medical Library Amino Acids Zwitterion In Water A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. Zwitterions in simple amino acid solutions. This again reflects the presence of the zwitterions. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules —. Amino Acids Zwitterion In Water.
From www.studocu.com
Amino acid worksheet 2 key AMINO ACID WORKSHEET Name Date Using the Amino Acids Zwitterion In Water This again reflects the presence of the zwitterions. Zwitterions in simple amino acid solutions. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. The zwitterionic state. Amino Acids Zwitterion In Water.
From warreninstitute.org
Calculating The Isoelectric Point Of Amino Acids And Zwitterions Amino Acids Zwitterion In Water An amino acid has both a basic amine group and an acidic carboxylic acid group. They act as buffer solutions as they resist any changes in ph when small. This again reflects the presence of the zwitterions. But, unlike simple amphoteric compounds that may only form. Because they carry both positive and. In water, the ionic attractions. A solution of. Amino Acids Zwitterion In Water.
From byjus.com
An amino acid under certain conditions has both positive and negative Amino Acids Zwitterion In Water When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. An amino acid has both a basic amine group and. Amino Acids Zwitterion In Water.
From cartoondealer.com
Zwitterion Forms Of Amino Acids Science Vector Illustration Diagram Amino Acids Zwitterion In Water In water, the ionic attractions. An amino acid has both a basic amine group and an acidic carboxylic acid group. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. Zwitterions in simple amino acid solutions. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid. Amino Acids Zwitterion In Water.
From chem.libretexts.org
23.4 Proteins and Amino Acids Chemistry LibreTexts Amino Acids Zwitterion In Water When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. But, unlike simple amphoteric compounds that may only form. In water, the ionic attractions. Zwitterions in simple amino acid. Amino Acids Zwitterion In Water.
From chemistry.com.pk
A Brief Introduction of Amino Acids The Building Blocks of Proteins Amino Acids Zwitterion In Water In water, the ionic attractions. Zwitterions in simple amino acid solutions. This again reflects the presence of the zwitterions. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. But, unlike simple amphoteric compounds that may only form. A solution of amino acids in water will. Amino Acids Zwitterion In Water.
From www.pinterest.jp
Zwitterion Its Nature, Occurrence, Ongoing Research Chemistry Amino Acids Zwitterion In Water In water, the ionic attractions. An amino acid has both a basic amine group and an acidic carboxylic acid group. This again reflects the presence of the zwitterions. Zwitterions in simple amino acid solutions. They act as buffer solutions as they resist any changes in ph when small. When an amino acid dissolves in water, the zwitterion interacts with h. Amino Acids Zwitterion In Water.
From www.chegg.com
Solved 9. What are the zwitterion and pl of amino acids and Amino Acids Zwitterion In Water When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. Zwitterions in simple amino acid solutions.. Amino Acids Zwitterion In Water.
From ar.inspiredpencil.com
Glutamic Acid Zwitterion Amino Acids Zwitterion In Water When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. An amino acid has both a basic amine group and. Amino Acids Zwitterion In Water.
From www.pinterest.com
Zwitterion state?! Review Charged Amino Acids brush up on amino acids Amino Acids Zwitterion In Water The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. An amino acid has both a basic amine group and an acidic carboxylic acid group. They act as buffer solutions as they resist any changes in ph when small. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. The. Amino Acids Zwitterion In Water.
From ar.inspiredpencil.com
Glutamic Acid Zwitterion Structure Amino Acids Zwitterion In Water But, unlike simple amphoteric compounds that may only form. An amino acid has both a basic amine group and an acidic carboxylic acid group. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and. Amino Acids Zwitterion In Water.
From slideplayer.com
Amino acids. ppt download Amino Acids Zwitterion In Water A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. When an amino acid contains both a plus and a minus charge in the backbone, it is called a. Amino Acids Zwitterion In Water.
From integrated-mcat.com
Amino Acid In Zwitterion Form Integrated MCAT Course Amino Acids Zwitterion In Water The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. They act as buffer solutions as they resist any changes in ph when small. But, unlike simple amphoteric compounds that may only form. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall. Amino Acids Zwitterion In Water.
From chemistnotes.com
zwitterion amino acid Isoelectric pH Chemistry Notes Amino Acids Zwitterion In Water When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. This again reflects the presence of the zwitterions. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. Zwitterions in simple amino acid solutions. They act as buffer solutions as they. Amino Acids Zwitterion In Water.
From www.chegg.com
Solved 1. Draw the zwitterion for the amino acids shown Amino Acids Zwitterion In Water Because they carry both positive and. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. They act as buffer solutions as they resist any changes in ph when small. When an amino acid contains both a plus and a. Amino Acids Zwitterion In Water.
From www.chegg.com
Solved 1. Draw the zwitterion for the amino acids shown Amino Acids Zwitterion In Water Because they carry both positive and. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Zwitterions in simple amino acid solutions. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. But, unlike simple amphoteric compounds that may only form.. Amino Acids Zwitterion In Water.
From slideplayer.com
Chapter 26 Amino Acids, Peptides, and Proteins ppt download Amino Acids Zwitterion In Water In water, the ionic attractions. Because they carry both positive and. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. Zwitterions in simple amino acid solutions. This again reflects the presence of the zwitterions. They act as buffer solutions as they resist any changes in ph when small. But, unlike simple amphoteric compounds that may only. Amino Acids Zwitterion In Water.
From sciencenotes.org
Zwitterion Definition and Examples Amino Acids Zwitterion In Water The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Zwitterions in simple amino acid solutions. An amino acid has both a basic amine group and an acidic carboxylic acid group. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base.. Amino Acids Zwitterion In Water.
From www.chegg.com
Solved The following amino acids are drawn in zwitterion Amino Acids Zwitterion In Water The zwitterion of an amino acid exists at a ph equal to the isoelectric point. But, unlike simple amphoteric compounds that may only form. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. This again reflects the presence of the zwitterions. An amino acid has. Amino Acids Zwitterion In Water.
From slideplayer.com
Biomolecule Amino Acids, Peptides, and Proteins Lecture 5 Dr. Aparna Amino Acids Zwitterion In Water The zwitterion of an amino acid exists at a ph equal to the isoelectric point. An amino acid has both a basic amine group and an acidic carboxylic acid group. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Zwitterions in simple amino. Amino Acids Zwitterion In Water.
From quizlet.com
What is a zwitterion? Under what conditions do amino acids b Quizlet Amino Acids Zwitterion In Water Zwitterions in simple amino acid solutions. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. They act as buffer solutions as they resist any changes in ph when small. But, unlike simple amphoteric compounds that may only. Amino Acids Zwitterion In Water.
From gbee.edu.vn
Isoelectric Points of Amino Acids (and How To Calculate Them) Gbee Amino Acids Zwitterion In Water In water, the ionic attractions. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. They act as buffer solutions as they resist any changes in ph when small. This again reflects the presence of the zwitterions. The zwitterionic state of amino acids plays. Amino Acids Zwitterion In Water.
From biochemden.com
What is ZwitterIon and Isoelectric Point? (Student notes) Amino Acids Zwitterion In Water They act as buffer solutions as they resist any changes in ph when small. An amino acid has both a basic amine group and an acidic carboxylic acid group. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. This again reflects the presence of the. Amino Acids Zwitterion In Water.
From slideplayer.com
Final Exam 104A Monday, May 10 800 1100 am 100 Noyes AQD,AQE Amino Acids Zwitterion In Water A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. In water, the ionic attractions. But, unlike simple amphoteric compounds that may only form. Because they carry both positive and. An amino acid has both a basic amine group and an acidic carboxylic acid group. When an amino acid dissolves in water, the. Amino Acids Zwitterion In Water.
From www.numerade.com
SOLVED Draw a tripeptide GlyPheAla 1] Write the structural formula Amino Acids Zwitterion In Water Because they carry both positive and. They act as buffer solutions as they resist any changes in ph when small. This again reflects the presence of the zwitterions. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. In water, the ionic attractions. Zwitterions in simple amino acid solutions. When an amino acid. Amino Acids Zwitterion In Water.
From www.expii.com
Zwitterions — Definition & Importance Expii Amino Acids Zwitterion In Water They act as buffer solutions as they resist any changes in ph when small. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. This again reflects the presence of the zwitterions. When an amino acid dissolves in water, the zwitterion interacts with h. Amino Acids Zwitterion In Water.
From www.slideserve.com
PPT Chapter 16 Amino Acids, Proteins, and Enzymes PowerPoint Amino Acids Zwitterion In Water When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. In water, the ionic attractions. An amino acid has both a basic amine group and an acidic carboxylic acid group. Because they carry both positive and. But, unlike simple amphoteric compounds that may only form. The. Amino Acids Zwitterion In Water.
From chemistryguru.com.sg
Deduce Zwitterion and Isoelectric Point of Amino Acids Amino Acids Zwitterion In Water This again reflects the presence of the zwitterions. In water, the ionic attractions. When an amino acid dissolves in water, the zwitterion interacts with h 2 o molecules — acting as both an acid and a base. The zwitterionic state of amino acids plays various roles, particularly in biochemical reactions. But, unlike simple amphoteric compounds that may only form. The. Amino Acids Zwitterion In Water.
From slideplayer.com
AMINO ACIDS. ppt download Amino Acids Zwitterion In Water In water, the ionic attractions. A solution of amino acids in water will exist as zwitterions with both acidic and basic properties. Zwitterions in simple amino acid solutions. The zwitterion of an amino acid exists at a ph equal to the isoelectric point. Because they carry both positive and. The zwitterionic state of amino acids plays various roles, particularly in. Amino Acids Zwitterion In Water.