Magnesium Sulfate Kinetics . In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching.
from studylib.net
Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from.
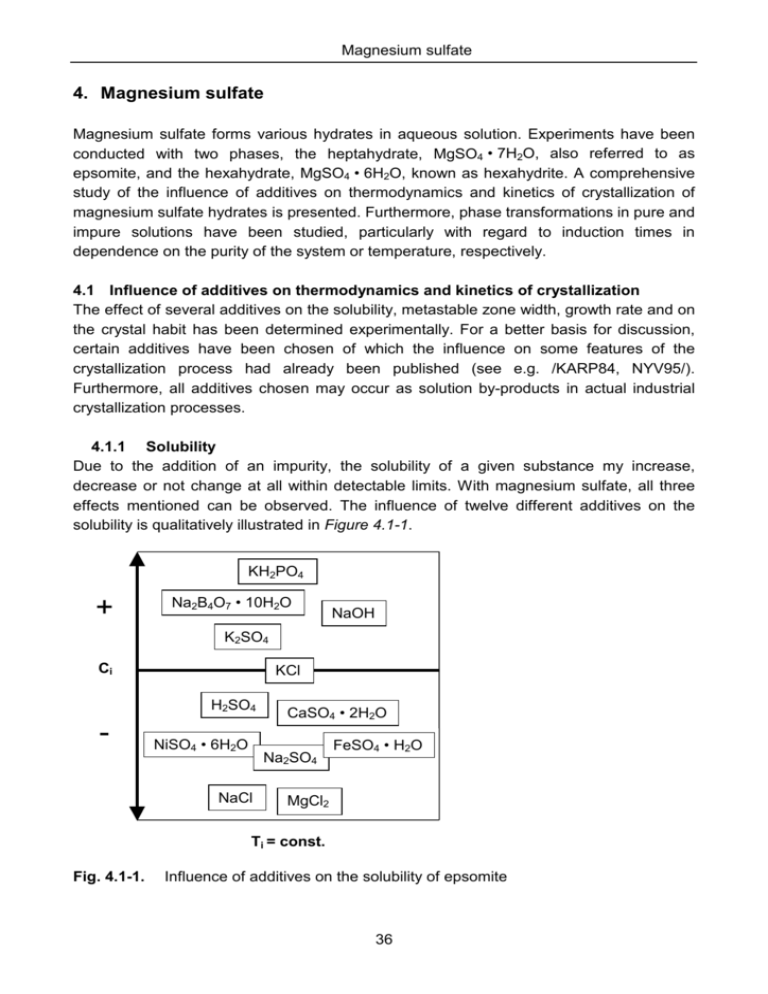
4. Magnesium sulfate
Magnesium Sulfate Kinetics Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from.
From www.mcguff.com
Magnesium Sulfate 50, 500mg/mL, SDV, 20mL Vial McGuff Magnesium Sulfate Kinetics Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. The effects of column leaching. Magnesium Sulfate Kinetics.
From vizda886.en.made-in-china.com
Premium Potassium Magnesium Sulfate Granular Fertilizer Fertilizer Magnesium Sulfate Kinetics In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Crystallization kinetics of magnesium. Magnesium Sulfate Kinetics.
From cartoondealer.com
Magnesium Sulfate, Salt, Molecular Structures, 3d Model, Structural Magnesium Sulfate Kinetics In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. Upon deriving the equation regulating the. Magnesium Sulfate Kinetics.
From www.researchgate.net
(PDF) Mechanical properties, thermal stability, and thermal degradation Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. In. Magnesium Sulfate Kinetics.
From hpnutrients.com
Magnesium Sulfate (25 lb bag) Hybrid Performance Nutrients Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Upon deriving the equation regulating the. Magnesium Sulfate Kinetics.
From www.netmeds.com
Buy MAGNESIUM SULPHATE Injection 2ml Online at Upto 25 OFF Netmeds Magnesium Sulfate Kinetics Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature. Magnesium Sulfate Kinetics.
From www.researchgate.net
Titration volume of magnesium sulfate versus pH change Download Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching.. Magnesium Sulfate Kinetics.
From journals.sagepub.com
Magnesium Sulfate Reduces CarrageenanInduced Rat Paw Inflammatory Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from.. Magnesium Sulfate Kinetics.
From kingmariot.com
500g Magnesium Sulphate DRIED Extra Pure Magnesium (II) Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: In this context, high temperature crystallization removes. Magnesium Sulfate Kinetics.
From dailymed.nlm.nih.gov
DailyMed MAGNESIUM SULFATE magnesium sulfate in water for injection Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Recently, the magnesium. Magnesium Sulfate Kinetics.
From onlinelibrary.wiley.com
Mechanical properties, thermal stability, and thermal degradation Magnesium Sulfate Kinetics Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this paper, magnesium sulfate was used as a. Magnesium Sulfate Kinetics.
From fda.report
MAGNESIUM SULFATE magnesium sulfate in water for injection Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. The effects. Magnesium Sulfate Kinetics.
From nexgenvetrx.com
Magnesium Sulfate 500 Mg/Ml Equine Sedation & Calming For Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. The effects. Magnesium Sulfate Kinetics.
From www.katyayaniorganics.com
Magnesium Sulfate / Epsom salt Katyayani Organics Magnesium Sulfate Kinetics Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. The effects of column leaching conditions, such as the concentration. Magnesium Sulfate Kinetics.
From www.aclsonline.us
Magnesium Sulfate in ACLS Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Recently, the magnesium sulfate thermal decomposition has been. Magnesium Sulfate Kinetics.
From www.studypool.com
SOLUTION Reaction Magnesium Sulfate 123 Studypool Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching.. Magnesium Sulfate Kinetics.
From www.researchgate.net
(PDF) Crystallization of Vitreous Magnesium Sulfate Hydrate Magnesium Sulfate Kinetics Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: The effects of column leaching conditions, such as the. Magnesium Sulfate Kinetics.
From jamiecooke.blogspot.com
Magnesium Sulfate Production Cost and Price Trends 20232028 Plant Magnesium Sulfate Kinetics In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature. Magnesium Sulfate Kinetics.
From www.lazada.co.th
Magnesium Sulfate แมกนีเซียมซัลเฟต (ดีเกลือฝรั่ง) ( ใช้เป็นสารเร่ง Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Recently, the magnesium sulfate thermal decomposition has. Magnesium Sulfate Kinetics.
From shopee.co.id
Jual ( MERCK ) Magnesium Sulfate Heptahydrate / Magnesium Sulfat Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Recently, the magnesium sulfate thermal decomposition has been reported as a. Magnesium Sulfate Kinetics.
From www.mcguffmedical.com
Magnesium Sulfate 50, 500mg/mL, SDV McGuff Medical Products Magnesium Sulfate Kinetics Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the In this context, high temperature crystallization removes magnesium,. Magnesium Sulfate Kinetics.
From geckogrow.ca
Magnesium Sulfate (Epsom Salt) 25 kg BULK Gecko Grow Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this context, high temperature crystallization removes. Magnesium Sulfate Kinetics.
From www.etsy.com
Magnesium Sulfate Protocol Sheet OB Nurse Magnesium Protocol Etsy Magnesium Sulfate Kinetics Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. In this context, high temperature crystallization removes magnesium, with a concentration of. Magnesium Sulfate Kinetics.
From rawchem2021.en.made-in-china.com
MgSO Series Magnesium Sulfate 7H2O for Industrial Grade China 10034 Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Upon deriving the equation regulating the leaching kinetics. Magnesium Sulfate Kinetics.
From journals.sagepub.com
Magnesium Sulfate Reduces CarrageenanInduced Rat Paw Inflammatory Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the. Magnesium Sulfate Kinetics.
From fivestarchemicals.com
Magnesium Sulfate Magnesium Sulfate Kinetics In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. The effects of column leaching conditions,. Magnesium Sulfate Kinetics.
From www.worldchemical.co.th
แมกนีเซียม ซัลเฟต (Magnesium Sulphate) ไทย World Chemical Group Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the In this context, high. Magnesium Sulfate Kinetics.
From www.researchgate.net
Time profiles of the effects of magnesium sulfate (MgSO4) and saline on Magnesium Sulfate Kinetics Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: In this context, high temperature crystallization removes magnesium, with a concentration of 8. Magnesium Sulfate Kinetics.
From www.researchgate.net
Solubility of α and β calcium sulfate hemihydrate and calcium sulfate Magnesium Sulfate Kinetics In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature crystallization removes magnesium, with a concentration of 8. Magnesium Sulfate Kinetics.
From journals.sagepub.com
Magnesium Sulfate Reduces CarrageenanInduced Rat Paw Inflammatory Magnesium Sulfate Kinetics Crystallization kinetics of magnesium sulphate monohydrate was studied at three different temperatures: In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In this context, high temperature crystallization removes magnesium, with a concentration of 8. Magnesium Sulfate Kinetics.
From eucogen.com
Eucogen Magnesium sulfate Kalceks 500mg/ml solution for injection Magnesium Sulfate Kinetics In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the In this paper, magnesium sulfate was used as a lixiviant to recover rare earth from kaolin. Recently, the magnesium sulfate. Magnesium Sulfate Kinetics.
From www.researchgate.net
(PDF) An Experimental Study on the of Leaching IonAdsorbed Magnesium Sulfate Kinetics In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. In. Magnesium Sulfate Kinetics.
From studylib.net
4. Magnesium sulfate Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching.. Magnesium Sulfate Kinetics.
From www.studocu.com
Magnesium sulfate study magnesium sulfate MIMS Class Laxatives Magnesium Sulfate Kinetics The effects of column leaching conditions, such as the concentration of magnesium sulfate, liquid/solid ratio, flow rate, and ph of the Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles.. Magnesium Sulfate Kinetics.
From lightssirensactionems.com
Magnesium Sulfate in EMS A Super Electrolyte Lights Sirens Action EMS Magnesium Sulfate Kinetics In this context, high temperature crystallization removes magnesium, with a concentration of 8 g/l, as mgso4 · h2o from. Upon deriving the equation regulating the leaching kinetics on the basis of the ree “shrinking core model” during the leaching. Recently, the magnesium sulfate thermal decomposition has been reported as a potential unit operation in one of these cycles. Crystallization kinetics. Magnesium Sulfate Kinetics.