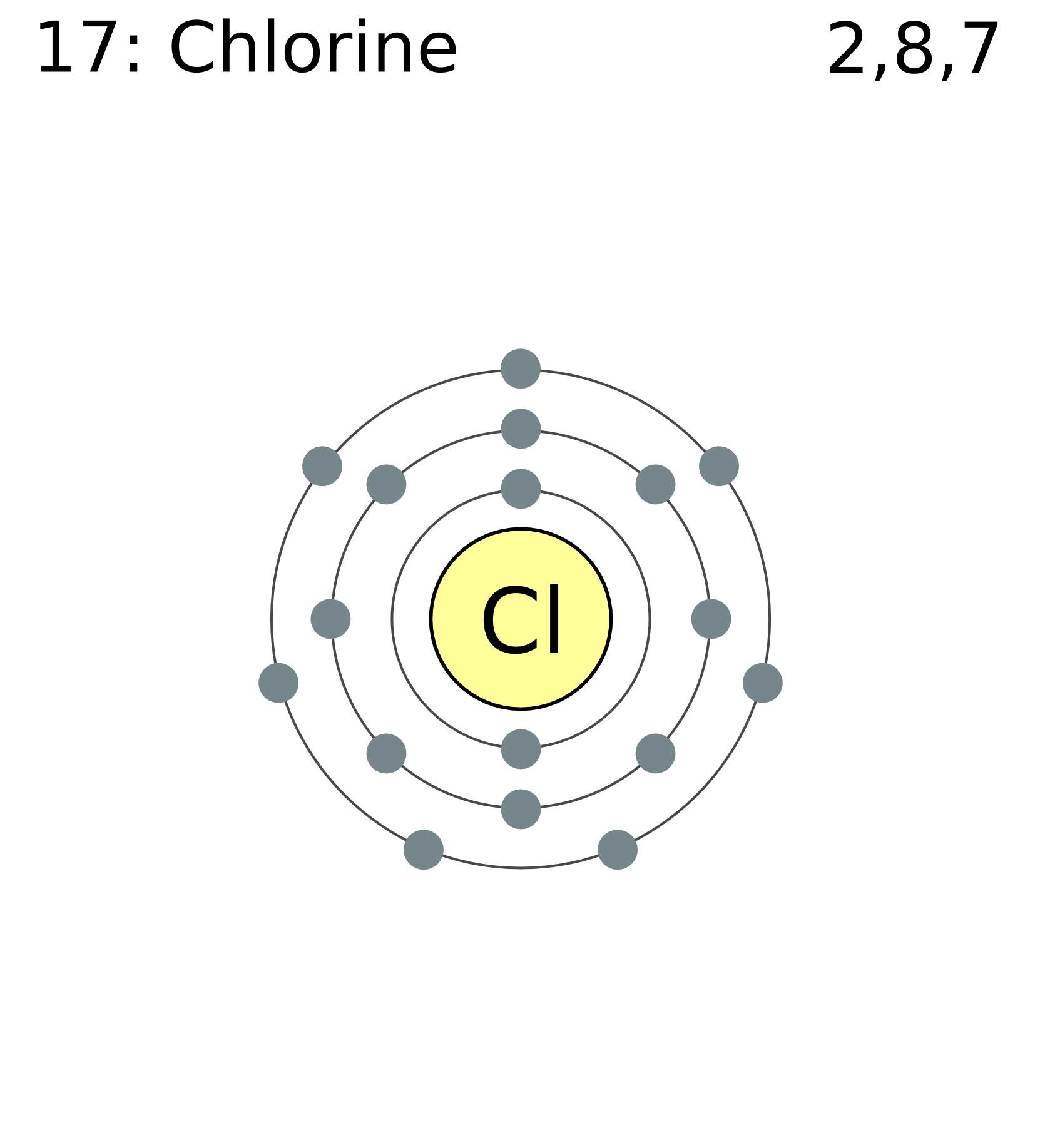
Chlorine Valence Electrons Lewis Structure . It tells us how electrons are organized. Determine the total number of valence electrons in the molecule or ion. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. The lewis dot structure for cl2 demonstrates how a. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The lewis structure indicates that each cl. cl2 lewis structure. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. Lewis structure is a simple depiction of valence shell electrons in a molecule. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line.
from commons.wikimedia.org
It tells us how electrons are organized. The lewis dot structure for cl2 demonstrates how a. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: cl2 lewis structure. Determine the total number of valence electrons in the molecule or ion. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. The lewis structure indicates that each cl. Lewis structure is a simple depiction of valence shell electrons in a molecule.
FileElectron shell 017 chlorine.png Wikimedia Commons
Chlorine Valence Electrons Lewis Structure The lewis dot structure for cl2 demonstrates how a. The lewis structure indicates that each cl. The lewis dot structure for cl2 demonstrates how a. cl2 lewis structure. It tells us how electrons are organized. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Lewis structure is a simple depiction of valence shell electrons in a molecule. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. Determine the total number of valence electrons in the molecule or ion.
From courses.lumenlearning.com
Lewis Symbols and Structures CHEM 1305 Introductory Chemistry Chlorine Valence Electrons Lewis Structure Lewis structure is a simple depiction of valence shell electrons in a molecule. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one.. Chlorine Valence Electrons Lewis Structure.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Valence Electrons Lewis Structure Lewis structure is a simple depiction of valence shell electrons in a molecule. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. The lewis dot structure for cl2 demonstrates how a. Determine the total number of valence electrons in the molecule or ion. cl2 lewis structure.. Chlorine Valence Electrons Lewis Structure.
From imgbin.com
Lewis Structure Chlorine Valence Electron Diagram PNG, Clipart, Atom Chlorine Valence Electrons Lewis Structure in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. Determine the total number of valence electrons in the molecule or ion. The. Chlorine Valence Electrons Lewis Structure.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Valence Electrons Lewis Structure the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. It tells us how electrons are organized. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. The lewis structure indicates that each. Chlorine Valence Electrons Lewis Structure.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Valence Electrons Lewis Structure the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. The lewis structure indicates that each cl. Determine the total number of valence electrons in the molecule or ion. The lewis dot structure for cl2 demonstrates how a. for example, when two chlorine atoms. Chlorine Valence Electrons Lewis Structure.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Valence Electrons Lewis Structure It tells us how electrons are organized. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. The lewis dot structure for cl2 demonstrates how a. the. Chlorine Valence Electrons Lewis Structure.
From pnghut.com
Atom Bohr Model Electron Configuration Chlorine Lewis Structure Chlorine Valence Electrons Lewis Structure the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. Lewis structure is a simple depiction of valence shell electrons in a molecule. The lewis structure indicates that each cl. for example, when two chlorine atoms form a chlorine molecule, they share one pair. Chlorine Valence Electrons Lewis Structure.
From www.chegg.com
Solved 10 Drag the tiles to the correct locations. Each tile Chlorine Valence Electrons Lewis Structure Determine the total number of valence electrons in the molecule or ion. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: cl2 lewis structure. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. The lewis structure indicates that. Chlorine Valence Electrons Lewis Structure.
From giowhypxq.blob.core.windows.net
Chlorine Expanded Valence Shell at Bradley Maiorano blog Chlorine Valence Electrons Lewis Structure The lewis dot structure for cl2 demonstrates how a. It tells us how electrons are organized. cl2 lewis structure. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing. Chlorine Valence Electrons Lewis Structure.
From www.slideserve.com
PPT Valence electrons, Lewis dot structures, and ionic charge Chlorine Valence Electrons Lewis Structure the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. It tells us how electrons are organized. The lewis dot structure for cl2 demonstrates how a. The lewis structure indicates that each cl. for example, when two chlorine atoms form a chlorine molecule, they. Chlorine Valence Electrons Lewis Structure.
From www.myxxgirl.com
Lewis Structure Chlorine Valence Electron Diagram Png Clipart Atom My Chlorine Valence Electrons Lewis Structure The lewis structure indicates that each cl. Lewis structure is a simple depiction of valence shell electrons in a molecule. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. The lewis dot structure for cl2 demonstrates how a. the nitrogen atom (group 15) has 5 valence. Chlorine Valence Electrons Lewis Structure.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Valence Electrons Lewis Structure The lewis structure indicates that each cl. Lewis structure is a simple depiction of valence shell electrons in a molecule. Determine the total number of valence electrons in the molecule or ion. The lewis dot structure for cl2 demonstrates how a. It tells us how electrons are organized. cl2 lewis structure. in this lewis dot diagram for cl2,. Chlorine Valence Electrons Lewis Structure.
From circuitdiagramyont.z1.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Valence Electrons Lewis Structure The lewis dot structure for cl2 demonstrates how a. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Lewis structure is a simple depiction of valence shell electrons in a molecule. The lewis structure indicates that each cl. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom. Chlorine Valence Electrons Lewis Structure.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Valence Electrons Lewis Structure Lewis structure is a simple depiction of valence shell electrons in a molecule. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line.. Chlorine Valence Electrons Lewis Structure.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Valence Electrons Lewis Structure The lewis dot structure for cl2 demonstrates how a. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Lewis structure is a simple depiction of valence shell. Chlorine Valence Electrons Lewis Structure.
From hanenhuusholli.blogspot.com
Lewis Dot Diagram For Chlorine Hanenhuusholli Chlorine Valence Electrons Lewis Structure the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. It tells us how electrons are organized. Determine the total number of valence electrons in the molecule or ion. for example, when two chlorine atoms form a chlorine molecule, they share one pair of. Chlorine Valence Electrons Lewis Structure.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Valence Electrons Lewis Structure in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. The lewis structure indicates that each cl. Lewis structure is a simple depiction of valence shell electrons in a molecule. the lewis structure indicates that each cl atom has three pairs of electrons that are not used. Chlorine Valence Electrons Lewis Structure.
From courses.lumenlearning.com
Lewis Symbols and Structures CHEM 1305 Introductory Chemistry Chlorine Valence Electrons Lewis Structure It tells us how electrons are organized. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. Lewis structure is a simple depiction of valence shell electrons in a molecule. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing. Chlorine Valence Electrons Lewis Structure.
From www.ck12.org
Valence Electrons CK12 Foundation Chlorine Valence Electrons Lewis Structure It tells us how electrons are organized. Lewis structure is a simple depiction of valence shell electrons in a molecule. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. the lewis structure indicates that each cl atom has three pairs of electrons that. Chlorine Valence Electrons Lewis Structure.
From www.myxxgirl.com
Chlorine Orbital Diagram Electron Configuration And Valence Electrons Chlorine Valence Electrons Lewis Structure in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. The lewis dot structure for cl2 demonstrates how a. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. the nitrogen atom. Chlorine Valence Electrons Lewis Structure.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Valence Electrons Lewis Structure It tells us how electrons are organized. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. cl2 lewis structure. Determine the total number of valence electrons in the molecule or ion. the nitrogen atom (group 15) has 5 valence electrons and each. Chlorine Valence Electrons Lewis Structure.
From chemistry291.blogspot.com
Cl2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar Chlorine Valence Electrons Lewis Structure in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. cl2 lewis structure. Lewis structure is a simple depiction of valence shell electrons in a molecule. Determine the total number of valence electrons in the molecule or ion. It tells us how electrons are organized. The lewis. Chlorine Valence Electrons Lewis Structure.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Valence Electrons Lewis Structure It tells us how electrons are organized. Determine the total number of valence electrons in the molecule or ion. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17). Chlorine Valence Electrons Lewis Structure.
From chemistry291.blogspot.com
【2 Steps】Cl2 Lewis Structure Lewis Dot Structure for Chlorine(Cl Chlorine Valence Electrons Lewis Structure cl2 lewis structure. It tells us how electrons are organized. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. Lewis structure is a simple depiction of valence shell electrons in a molecule. in this lewis dot diagram for cl2, each chlorine atom. Chlorine Valence Electrons Lewis Structure.
From socratic.org
What is the Lewis electrondot structure for a molecule of hydrogen Chlorine Valence Electrons Lewis Structure Determine the total number of valence electrons in the molecule or ion. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. Lewis structure is a simple depiction of valence shell electrons in a molecule. the lewis structure indicates that each cl atom has. Chlorine Valence Electrons Lewis Structure.
From brainly.com
Chloramine has the chemical formula NH2Cl. Nitrogen has five valence Chlorine Valence Electrons Lewis Structure for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: It tells us how electrons are organized. cl2 lewis structure. The lewis dot structure for cl2 demonstrates how a. The lewis structure indicates that each cl. Lewis structure is a simple depiction of valence shell electrons in a molecule. the lewis. Chlorine Valence Electrons Lewis Structure.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Valence Electrons Lewis Structure The lewis structure indicates that each cl. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: Determine the total number of valence electrons in the molecule or ion. It tells. Chlorine Valence Electrons Lewis Structure.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Valence Electrons Lewis Structure Determine the total number of valence electrons in the molecule or ion. The lewis structure indicates that each cl. Lewis structure is a simple depiction of valence shell electrons in a molecule. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. in this. Chlorine Valence Electrons Lewis Structure.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Valence Electrons Lewis Structure cl2 lewis structure. Determine the total number of valence electrons in the molecule or ion. the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in. Chlorine Valence Electrons Lewis Structure.
From www.chemtopper.com
How to Draw Lewis Dot Structure Online Chemistry Tutor Chlorine Valence Electrons Lewis Structure cl2 lewis structure. The lewis dot structure for cl2 demonstrates how a. Determine the total number of valence electrons in the molecule or ion. Lewis structure is a simple depiction of valence shell electrons in a molecule. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line.. Chlorine Valence Electrons Lewis Structure.
From www.youtube.com
How many valence electrons does chlorine have?How to find the valence Chlorine Valence Electrons Lewis Structure It tells us how electrons are organized. cl2 lewis structure. Determine the total number of valence electrons in the molecule or ion. The lewis structure indicates that each cl. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. Lewis structure is a simple depiction of valence. Chlorine Valence Electrons Lewis Structure.
From brainly.in
[Expert Verified] write electron dot diagram for chlorine and Calcium Chlorine Valence Electrons Lewis Structure The lewis structure indicates that each cl. The lewis dot structure for cl2 demonstrates how a. Determine the total number of valence electrons in the molecule or ion. the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. It tells us how electrons are organized.. Chlorine Valence Electrons Lewis Structure.
From draweasy8.blogspot.com
Hclo4 Lewis Structure Resonance Draw Easy Chlorine Valence Electrons Lewis Structure cl2 lewis structure. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. The lewis structure indicates that each cl. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: The lewis dot structure for cl2 demonstrates how a. . Chlorine Valence Electrons Lewis Structure.
From ar.inspiredpencil.com
Lewis Structure For Chlorine Ion Chlorine Valence Electrons Lewis Structure the nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26. Determine the total number of valence electrons in the molecule or ion. in this lewis dot diagram for cl2, each chlorine atom has seven dots, representing its seven valence electrons, and the line. . Chlorine Valence Electrons Lewis Structure.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Valence Electrons Lewis Structure the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one. It tells us how electrons are organized. for example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons: the nitrogen atom (group 15) has 5 valence electrons and. Chlorine Valence Electrons Lewis Structure.