Rate Of Diffusion Experiment . Mix two gases to explore diffusion! Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. One factor that can affect the rate of diffusion is the size of the molecule. In this activity, we will be observing. Larger molecules tend to move more slowly than smaller molecules. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this experiment, students will. With higher temperatures, particles are moving faster so they collide more often. So the higher the temperature, the higher the diffusion rate. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion You will investigate two dyes,. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid gel. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold.
from www.chegg.com
So the higher the temperature, the higher the diffusion rate. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. With higher temperatures, particles are moving faster so they collide more often. Larger molecules tend to move more slowly than smaller molecules. You will investigate two dyes,. One factor that can affect the rate of diffusion is the size of the molecule. Mix two gases to explore diffusion! In this activity, we will be observing.
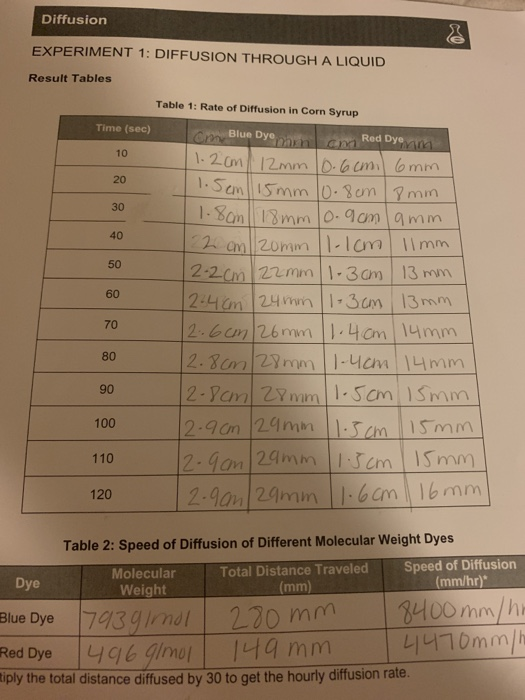
Solved Diffusion EXPERIMENT 1 DIFFUSION THROUGH A LIQUID
Rate Of Diffusion Experiment Mix two gases to explore diffusion! With higher temperatures, particles are moving faster so they collide more often. You will investigate two dyes,. Larger molecules tend to move more slowly than smaller molecules. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. One factor that can affect the rate of diffusion is the size of the molecule. In this experiment, students will. Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. Mix two gases to explore diffusion! In this activity, we will be observing. So the higher the temperature, the higher the diffusion rate. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid gel.
From www.numerade.com
SOLVED DIFFUSION EFFECT OF DENSITY OF MEDIA ON THE RATE OF DIFFUSION Rate Of Diffusion Experiment The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. With higher temperatures, particles are moving faster so they collide more often. You will investigate two dyes,. Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. In this experiment, students will. In this. Rate Of Diffusion Experiment.
From www.slideserve.com
PPT Solids, liquids and gases PowerPoint Presentation, free download Rate Of Diffusion Experiment An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion Mix two gases to explore diffusion! You will investigate two dyes,. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this activity, we will. Rate Of Diffusion Experiment.
From www.slideserve.com
PPT Graham’s Law of Diffusion PowerPoint Presentation ID4693447 Rate Of Diffusion Experiment Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. So the higher the temperature, the higher the diffusion rate. You will investigate two dyes,. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement.. Rate Of Diffusion Experiment.
From www.chegg.com
Solved Diffusion EXPERIMENT 1 DIFFUSION THROUGH A LIQUID Rate Of Diffusion Experiment So the higher the temperature, the higher the diffusion rate. Mix two gases to explore diffusion! With higher temperatures, particles are moving faster so they collide more often. In this activity, we will be observing. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid gel. Using food coloring. Rate Of Diffusion Experiment.
From www.slideserve.com
PPT Diffusion and Osmosis PowerPoint Presentation, free download ID Rate Of Diffusion Experiment With higher temperatures, particles are moving faster so they collide more often. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion Mix two gases to explore diffusion! Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion.. Rate Of Diffusion Experiment.
From www.visionlearning.com
Visionlearning Chemistry Diffusion I Rate Of Diffusion Experiment Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. Larger molecules tend to move more slowly than smaller molecules. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion One factor that can affect the rate of. Rate Of Diffusion Experiment.
From www.chegg.com
Solved Lab 6 Diffusion Data Sheet Record your hypothesis for Rate Of Diffusion Experiment In this experiment, students will. Larger molecules tend to move more slowly than smaller molecules. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. An example of how to set up an experiment to investigate the effect of changing surface area to volume. Rate Of Diffusion Experiment.
From www.youtube.com
Diffusion Rates YouTube Rate Of Diffusion Experiment In this experiment, students will. One factor that can affect the rate of diffusion is the size of the molecule. Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. In this activity, we will be observing. The purpose of this experiment is to determine the relationship between molecular weight and the rate. Rate Of Diffusion Experiment.
From magicgouveiaswasher.z21.web.core.windows.net
Effect Of Temperature On Diffusion Experiment Rate Of Diffusion Experiment Mix two gases to explore diffusion! Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. In this activity, we will be observing. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid. Rate Of Diffusion Experiment.
From www.studocu.com
How temperature affects the rate of diffusion [experiment] Rate of Rate Of Diffusion Experiment With higher temperatures, particles are moving faster so they collide more often. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion You will investigate two dyes,. In this activity, we will be observing. Larger molecules tend to move more slowly than smaller molecules. In. Rate Of Diffusion Experiment.
From studymind.co.uk
Investigating Transport Across Membranes (Alevel Biology) Study Mind Rate Of Diffusion Experiment One factor that can affect the rate of diffusion is the size of the molecule. With higher temperatures, particles are moving faster so they collide more often. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this experiment, students will. So the higher the temperature, the higher the diffusion. Rate Of Diffusion Experiment.
From wilsonevescience.blogspot.com
Practical Science Teaching Investigating diffusion at different Rate Of Diffusion Experiment Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. Larger molecules tend to move more slowly than smaller molecules. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid gel. An example of how to set up an experiment to investigate. Rate Of Diffusion Experiment.
From www.studocu.com
Lab 04 Part 2 Diffusion Effect of Co...n the Rate of Diffusion in a Rate Of Diffusion Experiment With higher temperatures, particles are moving faster so they collide more often. One factor that can affect the rate of diffusion is the size of the molecule. So the higher the temperature, the higher the diffusion rate. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both. Rate Of Diffusion Experiment.
From curriculum.newvisions.org
Diffusion Across a Membrane Prediction vs Results Tool New Visions Rate Of Diffusion Experiment The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. In this activity, we will be observing. Using food coloring as our solute, or material to be dissolved, we will watch and observe the. Rate Of Diffusion Experiment.
From www.savemyexams.com
Practical Investigating the Rate of Diffusion OCR A Level Biology Rate Of Diffusion Experiment Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. Mix two gases to explore diffusion! With higher temperatures, particles are moving faster so they collide more often. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion. Rate Of Diffusion Experiment.
From www.visionlearning.com
Diffusion I Chemistry Visionlearning Rate Of Diffusion Experiment With higher temperatures, particles are moving faster so they collide more often. Larger molecules tend to move more slowly than smaller molecules. In this activity, we will be observing. So the higher the temperature, the higher the diffusion rate. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid. Rate Of Diffusion Experiment.
From www.vrogue.co
Rate Of Diffusion Lab My E Portfolio vrogue.co Rate Of Diffusion Experiment The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. One factor that can affect the rate of diffusion is the size of the molecule. Mix two gases to explore diffusion! Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. Larger molecules tend. Rate Of Diffusion Experiment.
From www.youtube.com
How to Demonstrate Diffusion with Hot and Cold Water (Brownian Motion Rate Of Diffusion Experiment In this experiment, students will. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. Larger molecules tend to move more slowly than smaller molecules. Mix two gases to explore diffusion! Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion. Rate Of Diffusion Experiment.
From www.chegg.com
Solved Diffusion EXPERIMENT 1 DIFFUSION THROUGH A LIQUID Rate Of Diffusion Experiment Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion In this experiment, students will. Larger molecules tend to. Rate Of Diffusion Experiment.
From byjus.com
Which factor does not affect the rate of diffusion? Rate Of Diffusion Experiment One factor that can affect the rate of diffusion is the size of the molecule. With higher temperatures, particles are moving faster so they collide more often. So the higher the temperature, the higher the diffusion rate. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both. Rate Of Diffusion Experiment.
From www.pinterest.com.au
A lab that teaches osmosis, diffusion, concentration gradients Rate Of Diffusion Experiment Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. In this experiment, students will. Larger molecules tend to move more slowly than smaller molecules. Mix two gases to explore diffusion! With higher temperatures, particles are moving faster so they collide more often. The rate of diffusion is dependent upon the temperature of. Rate Of Diffusion Experiment.
From www.savemyexams.com
Practical Investigating the Rate of Diffusion OCR A Level Biology Rate Of Diffusion Experiment In this activity, we will be observing. Mix two gases to explore diffusion! With higher temperatures, particles are moving faster so they collide more often. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. Larger molecules tend to move more slowly than smaller molecules. The purpose of this experiment is. Rate Of Diffusion Experiment.
From www.slideserve.com
PPT Passive Transport What can move into and out of cells? PowerPoint Rate Of Diffusion Experiment Larger molecules tend to move more slowly than smaller molecules. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this activity, we will be observing. With higher temperatures, particles are moving faster so they collide more often. Experiment with concentration, temperature, mass, and radius and determine how these factors. Rate Of Diffusion Experiment.
From www.coursehero.com
[Solved] Data Sheet Experiment 1 Data Sheet Table 1 Rate of Diffusion Rate Of Diffusion Experiment Mix two gases to explore diffusion! Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. You will investigate two dyes,. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. In this activity, we will be. Rate Of Diffusion Experiment.
From thescienceteacher.co.uk
Diffusion teaching resources the science teacher Rate Of Diffusion Experiment The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this activity, we will be observing. With higher temperatures, particles are moving faster so they collide more often. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid gel.. Rate Of Diffusion Experiment.
From www.numerade.com
SOLVED Q 4. An experiment was set up to compare the rate (diffusion Rate Of Diffusion Experiment In this activity, we will be observing. In this experiment, students will. So the higher the temperature, the higher the diffusion rate. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion Using food coloring as our solute, or material to be dissolved, we will. Rate Of Diffusion Experiment.
From www.youtube.com
Factors that Affect Diffusion Rate YouTube Rate Of Diffusion Experiment Mix two gases to explore diffusion! So the higher the temperature, the higher the diffusion rate. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this activity, we will be observing. Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. Using. Rate Of Diffusion Experiment.
From www.youtube.com
Factors Affecting the Rate of Diffusion GCSE Biology YouTube Rate Of Diffusion Experiment Larger molecules tend to move more slowly than smaller molecules. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion So the higher the temperature, the. Rate Of Diffusion Experiment.
From www.youtube.com
3C lab effect of density of media on the rate of diffusion YouTube Rate Of Diffusion Experiment With higher temperatures, particles are moving faster so they collide more often. In this experiment, students will. An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion You will investigate two dyes,. Using food coloring as our solute, or material to be dissolved, we will. Rate Of Diffusion Experiment.
From www.youtube.com
Diffusion in gases YouTube Rate Of Diffusion Experiment Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. With higher temperatures, particles are moving faster so they collide more often. Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. The purpose of this experiment. Rate Of Diffusion Experiment.
From www.numerade.com
SOLVED In this experiment, we will measure the diffusion rates of Rate Of Diffusion Experiment The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. In this activity, we will be observing. One factor that can affect the rate of diffusion is the size of the molecule. You will investigate two dyes,. Larger molecules tend to move more slowly than smaller molecules. So the higher the. Rate Of Diffusion Experiment.
From byjus.com
Give a simple activity/experiment to show that the rate of diffusion Rate Of Diffusion Experiment An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion With higher temperatures, particles are moving faster so they collide more often. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid gel. So. Rate Of Diffusion Experiment.
From studylib.net
rate of diffusion agar experiment Rate Of Diffusion Experiment Experiment with concentration, temperature, mass, and radius and determine how these factors affect the rate of diffusion. Mix two gases to explore diffusion! Larger molecules tend to move more slowly than smaller molecules. The rate of diffusion is dependent upon the temperature of a system, because higher temperatures indicate greater molecular movement. The purpose of this experiment is to determine. Rate Of Diffusion Experiment.
From www.slideserve.com
PPT Chapter 6 Diffusion PowerPoint Presentation, free download ID Rate Of Diffusion Experiment An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion In this activity, we will be observing. The purpose of this experiment is to determine the relationship between molecular weight and the rate of diffusion through a semisolid gel. Larger molecules tend to move more. Rate Of Diffusion Experiment.
From www.instructables.com
Simple Diffusion Experiment 12 Steps Instructables Rate Of Diffusion Experiment So the higher the temperature, the higher the diffusion rate. Larger molecules tend to move more slowly than smaller molecules. Using food coloring as our solute, or material to be dissolved, we will watch and observe the rate of diffusion occurring in both hot and cold. Experiment with concentration, temperature, mass, and radius and determine how these factors affect the. Rate Of Diffusion Experiment.