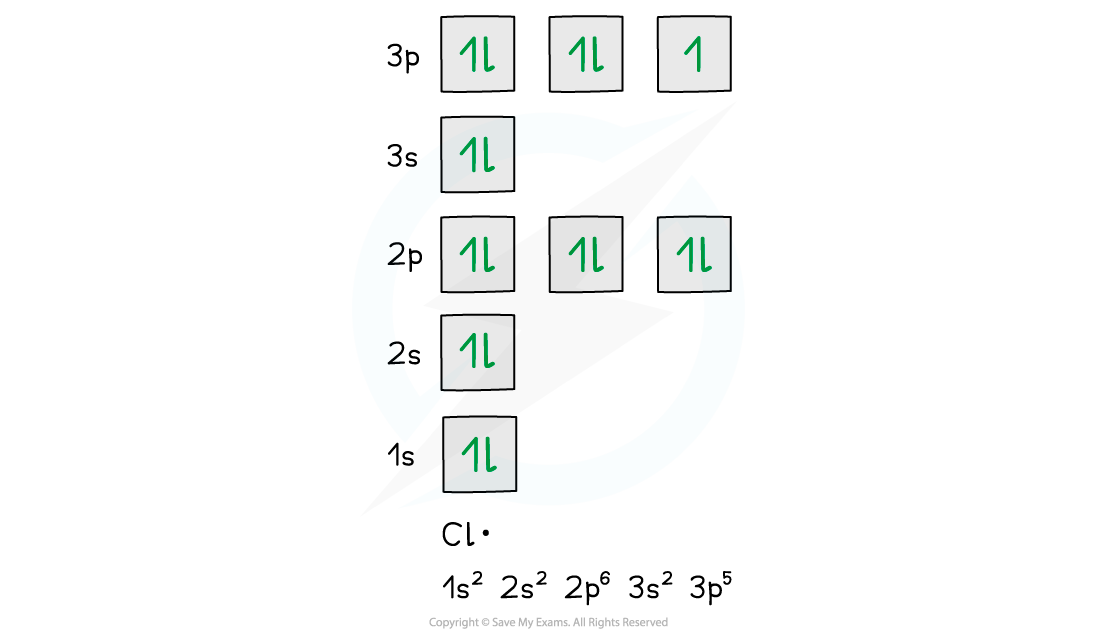
Chlorine Free Radical Electron Configuration . In ethane halogenation, a chlorine radical The chlorine molecule has a covalent bond joining the 2 chlorine. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. This reaction is called a chain reaction because, as we will see, homolytic. for example, a chlorine atom has the electronic configuration 2, 8, 7. This arrangement is highly unstable and the. That hydrogen atom only needs to bring one electron with it to. the chlorine radical removes a hydrogen atom from the methane. — the free chlorine atom is a radical with one unpaired electron. a radical is a species with an unpaired electron. an illustrative example of a free radical reaction is the chlorination of methane.
from www.linstitute.net
— the free chlorine atom is a radical with one unpaired electron. This reaction is called a chain reaction because, as we will see, homolytic. The chlorine molecule has a covalent bond joining the 2 chlorine. an illustrative example of a free radical reaction is the chlorination of methane. for example, a chlorine atom has the electronic configuration 2, 8, 7. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. In ethane halogenation, a chlorine radical the chlorine radical removes a hydrogen atom from the methane. That hydrogen atom only needs to bring one electron with it to. This arrangement is highly unstable and the.
CIE A Level Chemistry复习笔记1.1.8 Electron Configuration翰林国际教育
Chlorine Free Radical Electron Configuration That hydrogen atom only needs to bring one electron with it to. In ethane halogenation, a chlorine radical — the free chlorine atom is a radical with one unpaired electron. This arrangement is highly unstable and the. an illustrative example of a free radical reaction is the chlorination of methane. the chlorine radical removes a hydrogen atom from the methane. for example, a chlorine atom has the electronic configuration 2, 8, 7. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. That hydrogen atom only needs to bring one electron with it to. The chlorine molecule has a covalent bond joining the 2 chlorine. This reaction is called a chain reaction because, as we will see, homolytic. a radical is a species with an unpaired electron.
From www.youtube.com
Electron Configuration Diagram for Chlorine YouTube Chlorine Free Radical Electron Configuration This reaction is called a chain reaction because, as we will see, homolytic. — the free chlorine atom is a radical with one unpaired electron. In ethane halogenation, a chlorine radical the chlorine radical removes a hydrogen atom from the methane. a radical is a species with an unpaired electron. This arrangement is highly unstable and the.. Chlorine Free Radical Electron Configuration.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Free Radical Electron Configuration for example, a chlorine atom has the electronic configuration 2, 8, 7. a radical is a species with an unpaired electron. the chlorine radical removes a hydrogen atom from the methane. The chlorine molecule has a covalent bond joining the 2 chlorine. the three free radicals shown in the bottom panel of figure 1 are called. Chlorine Free Radical Electron Configuration.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Free Radical Electron Configuration — the free chlorine atom is a radical with one unpaired electron. In ethane halogenation, a chlorine radical This arrangement is highly unstable and the. That hydrogen atom only needs to bring one electron with it to. the chlorine radical removes a hydrogen atom from the methane. for example, a chlorine atom has the electronic configuration 2,. Chlorine Free Radical Electron Configuration.
From brainly.com
The diagram shows the electron configuration around the chlorine Chlorine Free Radical Electron Configuration This arrangement is highly unstable and the. This reaction is called a chain reaction because, as we will see, homolytic. for example, a chlorine atom has the electronic configuration 2, 8, 7. the chlorine radical removes a hydrogen atom from the methane. a radical is a species with an unpaired electron. — the free chlorine atom. Chlorine Free Radical Electron Configuration.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Free Radical Electron Configuration This arrangement is highly unstable and the. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. — the free chlorine atom is a radical with one unpaired electron. The chlorine molecule has a covalent bond joining the 2 chlorine. This reaction is called a chain reaction. Chlorine Free Radical Electron Configuration.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Free Radical Electron Configuration the chlorine radical removes a hydrogen atom from the methane. This reaction is called a chain reaction because, as we will see, homolytic. The chlorine molecule has a covalent bond joining the 2 chlorine. — the free chlorine atom is a radical with one unpaired electron. the three free radicals shown in the bottom panel of figure. Chlorine Free Radical Electron Configuration.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Free Radical Electron Configuration — the free chlorine atom is a radical with one unpaired electron. the chlorine radical removes a hydrogen atom from the methane. for example, a chlorine atom has the electronic configuration 2, 8, 7. The chlorine molecule has a covalent bond joining the 2 chlorine. the three free radicals shown in the bottom panel of figure. Chlorine Free Radical Electron Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Free Radical Electron Configuration a radical is a species with an unpaired electron. This arrangement is highly unstable and the. This reaction is called a chain reaction because, as we will see, homolytic. — the free chlorine atom is a radical with one unpaired electron. The chlorine molecule has a covalent bond joining the 2 chlorine. In ethane halogenation, a chlorine radical. Chlorine Free Radical Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Free Radical Electron Configuration the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. — the free chlorine atom is a radical with one unpaired electron. That hydrogen atom only needs to bring one electron with it to. In ethane halogenation, a chlorine radical This arrangement is highly unstable and the.. Chlorine Free Radical Electron Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Free Radical Electron Configuration That hydrogen atom only needs to bring one electron with it to. In ethane halogenation, a chlorine radical a radical is a species with an unpaired electron. The chlorine molecule has a covalent bond joining the 2 chlorine. — the free chlorine atom is a radical with one unpaired electron. the three free radicals shown in the. Chlorine Free Radical Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Free Radical Electron Configuration a radical is a species with an unpaired electron. This reaction is called a chain reaction because, as we will see, homolytic. the chlorine radical removes a hydrogen atom from the methane. The chlorine molecule has a covalent bond joining the 2 chlorine. — the free chlorine atom is a radical with one unpaired electron. This arrangement. Chlorine Free Radical Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Free Radical Electron Configuration the chlorine radical removes a hydrogen atom from the methane. an illustrative example of a free radical reaction is the chlorination of methane. The chlorine molecule has a covalent bond joining the 2 chlorine. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. This reaction. Chlorine Free Radical Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Free Radical Electron Configuration That hydrogen atom only needs to bring one electron with it to. for example, a chlorine atom has the electronic configuration 2, 8, 7. In ethane halogenation, a chlorine radical This arrangement is highly unstable and the. The chlorine molecule has a covalent bond joining the 2 chlorine. the chlorine radical removes a hydrogen atom from the methane.. Chlorine Free Radical Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Free Radical Electron Configuration for example, a chlorine atom has the electronic configuration 2, 8, 7. This reaction is called a chain reaction because, as we will see, homolytic. The chlorine molecule has a covalent bond joining the 2 chlorine. — the free chlorine atom is a radical with one unpaired electron. In ethane halogenation, a chlorine radical the chlorine radical. Chlorine Free Radical Electron Configuration.
From www.pinterest.com
Atom Chlorine This diagram shows the electron shell configuration for Chlorine Free Radical Electron Configuration That hydrogen atom only needs to bring one electron with it to. an illustrative example of a free radical reaction is the chlorination of methane. This reaction is called a chain reaction because, as we will see, homolytic. a radical is a species with an unpaired electron. the chlorine radical removes a hydrogen atom from the methane.. Chlorine Free Radical Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Free Radical Electron Configuration an illustrative example of a free radical reaction is the chlorination of methane. a radical is a species with an unpaired electron. for example, a chlorine atom has the electronic configuration 2, 8, 7. In ethane halogenation, a chlorine radical the chlorine radical removes a hydrogen atom from the methane. That hydrogen atom only needs to. Chlorine Free Radical Electron Configuration.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Free Radical Electron Configuration the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. — the free chlorine atom is a radical with one unpaired electron. This reaction is called a chain reaction because, as we will see, homolytic. an illustrative example of a free radical reaction is the chlorination. Chlorine Free Radical Electron Configuration.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Free Radical Electron Configuration This arrangement is highly unstable and the. In ethane halogenation, a chlorine radical the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. — the free chlorine atom is a radical with one unpaired electron. This reaction is called a chain reaction because, as we will see,. Chlorine Free Radical Electron Configuration.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Free Radical Electron Configuration That hydrogen atom only needs to bring one electron with it to. — the free chlorine atom is a radical with one unpaired electron. the chlorine radical removes a hydrogen atom from the methane. for example, a chlorine atom has the electronic configuration 2, 8, 7. a radical is a species with an unpaired electron. In. Chlorine Free Radical Electron Configuration.
From 88guru.com
Electron Configuration Rules, Example and Diagram 88Guru Chlorine Free Radical Electron Configuration for example, a chlorine atom has the electronic configuration 2, 8, 7. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. an illustrative example of a free radical reaction is the chlorination of methane. In ethane halogenation, a chlorine radical This arrangement is highly unstable. Chlorine Free Radical Electron Configuration.
From sciencenotes.org
Chlorine Facts Chlorine Free Radical Electron Configuration for example, a chlorine atom has the electronic configuration 2, 8, 7. The chlorine molecule has a covalent bond joining the 2 chlorine. This reaction is called a chain reaction because, as we will see, homolytic. a radical is a species with an unpaired electron. the chlorine radical removes a hydrogen atom from the methane. an. Chlorine Free Radical Electron Configuration.
From www.slideserve.com
PPT Free Radical Chlorination PowerPoint Presentation, free download Chlorine Free Radical Electron Configuration That hydrogen atom only needs to bring one electron with it to. This reaction is called a chain reaction because, as we will see, homolytic. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. for example, a chlorine atom has the electronic configuration 2, 8, 7.. Chlorine Free Radical Electron Configuration.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.1.8 Electron Configuration翰林国际教育 Chlorine Free Radical Electron Configuration In ethane halogenation, a chlorine radical — the free chlorine atom is a radical with one unpaired electron. The chlorine molecule has a covalent bond joining the 2 chlorine. an illustrative example of a free radical reaction is the chlorination of methane. That hydrogen atom only needs to bring one electron with it to. the chlorine radical. Chlorine Free Radical Electron Configuration.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Free Radical Electron Configuration an illustrative example of a free radical reaction is the chlorination of methane. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. The chlorine molecule has a covalent bond joining the 2 chlorine. This arrangement is highly unstable and the. for example, a chlorine atom. Chlorine Free Radical Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Free Radical Electron Configuration This arrangement is highly unstable and the. an illustrative example of a free radical reaction is the chlorination of methane. The chlorine molecule has a covalent bond joining the 2 chlorine. — the free chlorine atom is a radical with one unpaired electron. This reaction is called a chain reaction because, as we will see, homolytic. the. Chlorine Free Radical Electron Configuration.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Free Radical Electron Configuration — the free chlorine atom is a radical with one unpaired electron. In ethane halogenation, a chlorine radical the chlorine radical removes a hydrogen atom from the methane. for example, a chlorine atom has the electronic configuration 2, 8, 7. This arrangement is highly unstable and the. That hydrogen atom only needs to bring one electron with. Chlorine Free Radical Electron Configuration.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Free Radical Electron Configuration a radical is a species with an unpaired electron. an illustrative example of a free radical reaction is the chlorination of methane. This arrangement is highly unstable and the. In ethane halogenation, a chlorine radical — the free chlorine atom is a radical with one unpaired electron. the three free radicals shown in the bottom panel. Chlorine Free Radical Electron Configuration.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Free Radical Electron Configuration This reaction is called a chain reaction because, as we will see, homolytic. That hydrogen atom only needs to bring one electron with it to. for example, a chlorine atom has the electronic configuration 2, 8, 7. In ethane halogenation, a chlorine radical the chlorine radical removes a hydrogen atom from the methane. the three free radicals. Chlorine Free Radical Electron Configuration.
From www.transtutors.com
(Get Answer) What is the electron configuration for Cl, chlorine, and Chlorine Free Radical Electron Configuration The chlorine molecule has a covalent bond joining the 2 chlorine. That hydrogen atom only needs to bring one electron with it to. the chlorine radical removes a hydrogen atom from the methane. This arrangement is highly unstable and the. In ethane halogenation, a chlorine radical the three free radicals shown in the bottom panel of figure 1. Chlorine Free Radical Electron Configuration.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Free Radical Electron Configuration an illustrative example of a free radical reaction is the chlorination of methane. That hydrogen atom only needs to bring one electron with it to. the chlorine radical removes a hydrogen atom from the methane. a radical is a species with an unpaired electron. This reaction is called a chain reaction because, as we will see, homolytic.. Chlorine Free Radical Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Free Radical Electron Configuration This arrangement is highly unstable and the. an illustrative example of a free radical reaction is the chlorination of methane. This reaction is called a chain reaction because, as we will see, homolytic. a radical is a species with an unpaired electron. the chlorine radical removes a hydrogen atom from the methane. In ethane halogenation, a chlorine. Chlorine Free Radical Electron Configuration.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Free Radical Electron Configuration a radical is a species with an unpaired electron. That hydrogen atom only needs to bring one electron with it to. The chlorine molecule has a covalent bond joining the 2 chlorine. the three free radicals shown in the bottom panel of figure 1 are called chlorine atoms, hydroxyl radicals, and triphenylmethyl radicals. an illustrative example of. Chlorine Free Radical Electron Configuration.
From egpat.com
Lewis dot structure How to write? Chlorine Free Radical Electron Configuration This arrangement is highly unstable and the. This reaction is called a chain reaction because, as we will see, homolytic. the chlorine radical removes a hydrogen atom from the methane. an illustrative example of a free radical reaction is the chlorination of methane. — the free chlorine atom is a radical with one unpaired electron. In ethane. Chlorine Free Radical Electron Configuration.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Chlorine Free Radical Electron Configuration a radical is a species with an unpaired electron. for example, a chlorine atom has the electronic configuration 2, 8, 7. an illustrative example of a free radical reaction is the chlorination of methane. In ethane halogenation, a chlorine radical This arrangement is highly unstable and the. That hydrogen atom only needs to bring one electron with. Chlorine Free Radical Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Free Radical Electron Configuration the chlorine radical removes a hydrogen atom from the methane. an illustrative example of a free radical reaction is the chlorination of methane. This arrangement is highly unstable and the. In ethane halogenation, a chlorine radical This reaction is called a chain reaction because, as we will see, homolytic. for example, a chlorine atom has the electronic. Chlorine Free Radical Electron Configuration.