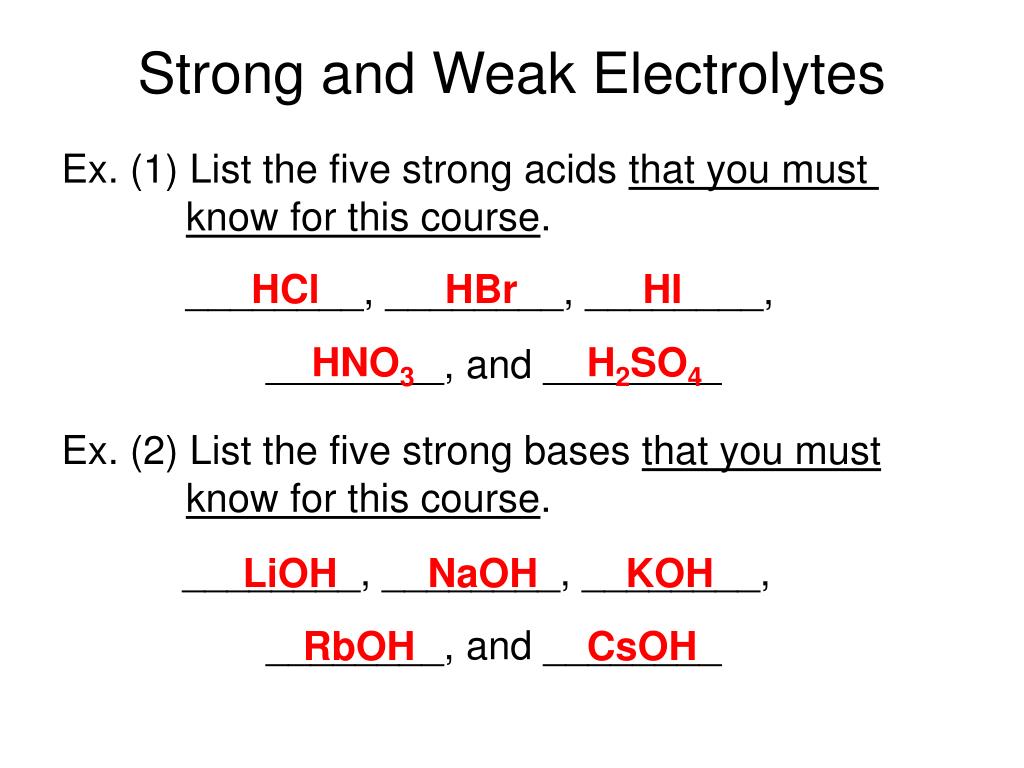
Lead Acetate Strong Or Weak Electrolyte . Weak acids are weak electrolytes, and most. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Lead (ii) acetate is soluble in. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Electrolytes are chemicals that break into ions (ionize) when. Strong electrolytes include the strong acids, strong bases, and salts. These chemicals completely dissociate into ions in aqueous solution.
from www.slideserve.com
These chemicals completely dissociate into ions in aqueous solution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Strong electrolytes include the strong acids, strong bases, and salts. Weak acids are weak electrolytes, and most. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Electrolytes are chemicals that break into ions (ionize) when. Lead (ii) acetate is soluble in.
PPT Strong and Weak Electrolytes PowerPoint Presentation, free
Lead Acetate Strong Or Weak Electrolyte Soluble salts tend to be strong electrolytes because they release charged ions into solution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Electrolytes are chemicals that break into ions (ionize) when. Lead (ii) acetate is soluble in. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Weak acids are weak electrolytes, and most. Strong electrolytes include the strong acids, strong bases, and salts. These chemicals completely dissociate into ions in aqueous solution. Salts are often strong electrolytes, and strong acids are always strong electrolytes.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Electrolytes are chemicals that break into ions (ionize) when. These chemicals completely dissociate into ions. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 Solution Chemistry and the Hydrosphere PowerPoint Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Electrolytes are chemicals that break into ions (ionize) when. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. These chemicals completely dissociate into ions in aqueous solution. Salts are often strong electrolytes, and strong acids are always strong electrolytes.. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Aqueous Reactions and Solution Stoichiometry PowerPoint Lead Acetate Strong Or Weak Electrolyte Soluble salts tend to be strong electrolytes because they release charged ions into solution. Weak acids are weak electrolytes, and most. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Strong electrolytes include the strong acids, strong bases, and salts. These chemicals completely dissociate into ions in aqueous solution. Electrolytes are chemicals that break into ions (ionize). Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. Weak acids are weak electrolytes, and most. Weak electrolytes less is more for these oddities which possess the. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Weak, Strong and Nonelectrolytes PowerPoint Presentation, free Lead Acetate Strong Or Weak Electrolyte Weak acids are weak electrolytes, and most. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Lead (ii) acetate is soluble in. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. These chemicals completely dissociate into ions in aqueous solution. Soluble salts tend to be. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Electrolytes are chemicals that break into ions (ionize) when. Lead (ii) acetate is soluble in. Weak acids are weak electrolytes, and most. Salts are often strong electrolytes, and strong acids are always strong electrolytes. These chemicals completely dissociate into ions in aqueous solution. Soluble salts tend to be strong electrolytes. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Electrolytes are chemicals that break into ions (ionize) when. Soluble salts tend to be strong electrolytes because they release charged ions into solution. These chemicals completely dissociate into ions in aqueous solution. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Weak electrolytes less is more for. Lead Acetate Strong Or Weak Electrolyte.
From slideplayer.com
AQUEOUS EQUILIBRIA AP Chapter ppt video online download Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Electrolytes are chemicals that break into ions (ionize) when. These chemicals completely dissociate into ions in aqueous solution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity. Lead Acetate Strong Or Weak Electrolyte.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Lead Acetate Strong Or Weak Electrolyte Salts are often strong electrolytes, and strong acids are always strong electrolytes. These chemicals completely dissociate into ions in aqueous solution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Electrolytes are chemicals that break into ions (ionize) when. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite. Lead Acetate Strong Or Weak Electrolyte.
From sharedocnow.blogspot.com
List Of Strong Electrolytes Chemistry sharedoc Lead Acetate Strong Or Weak Electrolyte Electrolytes are chemicals that break into ions (ionize) when. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Weak acids are weak electrolytes, and most. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. These chemicals completely. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter Nine Chemical Reactions in Aqueous Solutions PowerPoint Lead Acetate Strong Or Weak Electrolyte Salts are often strong electrolytes, and strong acids are always strong electrolytes. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. Electrolytes are chemicals that break into ions (ionize) when. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at. Lead Acetate Strong Or Weak Electrolyte.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Lead Acetate Strong Or Weak Electrolyte These chemicals completely dissociate into ions in aqueous solution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Strong electrolytes include the strong acids, strong bases, and salts. Lead (ii) acetate is soluble in. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Weak acids are weak electrolytes, and most. Weak electrolytes. Lead Acetate Strong Or Weak Electrolyte.
From www.numerade.com
SOLVED Table Solution NaCI Electrolyte (weak or strong) ornon Lead Acetate Strong Or Weak Electrolyte Salts are often strong electrolytes, and strong acids are always strong electrolytes. Weak acids are weak electrolytes, and most. Electrolytes are chemicals that break into ions (ionize) when. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Soluble salts tend to be strong electrolytes because they release charged. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Aqueous Reactions and Solution Stoichiometry PowerPoint Lead Acetate Strong Or Weak Electrolyte Electrolytes are chemicals that break into ions (ionize) when. Strong electrolytes include the strong acids, strong bases, and salts. These chemicals completely dissociate into ions in aqueous solution. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Soluble salts tend to be strong electrolytes because they release charged. Lead Acetate Strong Or Weak Electrolyte.
From sharedocnow.blogspot.com
List Of Strong And Weak Electrolytes sharedoc Lead Acetate Strong Or Weak Electrolyte Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. Weak acids are weak electrolytes, and most. These chemicals completely dissociate into ions in aqueous solution. Salts are often. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download Lead Acetate Strong Or Weak Electrolyte Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. These chemicals completely dissociate into ions in aqueous solution. Electrolytes are chemicals that break into ions (ionize) when. Weak. Lead Acetate Strong Or Weak Electrolyte.
From melissa-media.blogspot.com
Melissa Media Strong And Weak Electrolytes Lead Acetate Strong Or Weak Electrolyte These chemicals completely dissociate into ions in aqueous solution. Lead (ii) acetate is soluble in. Electrolytes are chemicals that break into ions (ionize) when. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Weak acids are weak electrolytes, and most. Strong electrolytes include the strong acids, strong bases,. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Electrolytes are chemicals that break into ions (ionize) when. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. These chemicals completely dissociate into ions. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Lead Acetate Strong Or Weak Electrolyte Soluble salts tend to be strong electrolytes because they release charged ions into solution. These chemicals completely dissociate into ions in aqueous solution. Weak acids are weak electrolytes, and most. Lead (ii) acetate is soluble in. Strong electrolytes include the strong acids, strong bases, and salts. Electrolytes are chemicals that break into ions (ionize) when. Salts are often strong electrolytes,. Lead Acetate Strong Or Weak Electrolyte.
From www.w3schools.blog
Strong and weak electrolytes W3schools Lead Acetate Strong Or Weak Electrolyte Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Electrolytes are chemicals that break into ions (ionize) when. Salts are often strong electrolytes, and strong acids are always strong electrolytes. These chemicals completely dissociate into ions in aqueous solution. Weak acids are weak electrolytes, and most. Lead (ii). Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID3839298 Lead Acetate Strong Or Weak Electrolyte Weak acids are weak electrolytes, and most. These chemicals completely dissociate into ions in aqueous solution. Electrolytes are chemicals that break into ions (ionize) when. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Strong electrolytes include the strong acids, strong bases, and salts. Lead (ii) acetate is soluble in. Soluble salts tend to be strong electrolytes. Lead Acetate Strong Or Weak Electrolyte.
From www.kentchemistry.com
Electrolytes Lead Acetate Strong Or Weak Electrolyte Weak acids are weak electrolytes, and most. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. These chemicals completely dissociate into ions in aqueous solution. Lead (ii) acetate is soluble in. Soluble salts tend to be. Lead Acetate Strong Or Weak Electrolyte.
From general.chemistrysteps.com
Strong and Weak Electrolytes Chemistry Steps Lead Acetate Strong Or Weak Electrolyte Soluble salts tend to be strong electrolytes because they release charged ions into solution. Weak acids are weak electrolytes, and most. Lead (ii) acetate is soluble in. Electrolytes are chemicals that break into ions (ionize) when. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Salts are often. Lead Acetate Strong Or Weak Electrolyte.
From www.animalia-life.club
Electrolyte Strength Chart Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Soluble salts tend to be strong electrolytes because they release charged ions into solution. These chemicals completely dissociate into ions in aqueous solution. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Electrolytes are chemicals that break into ions (ionize) when. Weak acids are weak electrolytes, and. Lead Acetate Strong Or Weak Electrolyte.
From mungfali.com
Electrolyte Chart Diagram Lead Acetate Strong Or Weak Electrolyte Weak acids are weak electrolytes, and most. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Strong electrolytes include the strong acids, strong bases, and salts. Lead (ii) acetate is soluble in. These chemicals. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT 24.6 The conductivities of electrolyte solutions PowerPoint Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Electrolytes are chemicals that break into ions (ionize) when. Lead (ii) acetate is soluble in. Weak acids are weak electrolytes, and most. Salts are often strong electrolytes, and strong acids are always strong electrolytes. These chemicals. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download Lead Acetate Strong Or Weak Electrolyte These chemicals completely dissociate into ions in aqueous solution. Strong electrolytes include the strong acids, strong bases, and salts. Electrolytes are chemicals that break into ions (ionize) when. Weak acids are weak electrolytes, and most. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Lead (ii) acetate is soluble in. Weak electrolytes less is more for these. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT II. Types of Electrolytes PowerPoint Presentation, free download Lead Acetate Strong Or Weak Electrolyte Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Strong electrolytes include the strong acids, strong bases, and salts. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Lead (ii) acetate is soluble in. Electrolytes are chemicals that break into ions (ionize) when. Weak acids. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID3839298 Lead Acetate Strong Or Weak Electrolyte Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Weak acids are weak electrolytes, and most.. Lead Acetate Strong Or Weak Electrolyte.
From pediaa.com
Difference Between Strong and Weak Electrolytes Definition Lead Acetate Strong Or Weak Electrolyte Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Electrolytes are chemicals that break into ions (ionize) when. Weak acids are weak electrolytes, and most. These chemicals completely dissociate into ions in aqueous solution. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Soluble salts. Lead Acetate Strong Or Weak Electrolyte.
From www.youtube.com
Electrolytes Strong Electrolytes, Weak Electrolytes, and Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Electrolytes are chemicals that break into ions (ionize) when. These chemicals completely dissociate into ions in aqueous solution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in.. Lead Acetate Strong Or Weak Electrolyte.
From www.numerade.com
SOLVED The compound lead(II) acetate is a strong electrolyte. Write Lead Acetate Strong Or Weak Electrolyte Strong electrolytes include the strong acids, strong bases, and salts. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Electrolytes are chemicals that break into ions (ionize) when. These chemicals completely dissociate into ions in aqueous solution. Salts are often strong electrolytes, and strong acids are always strong electrolytes. Weak acids are weak electrolytes, and. Lead Acetate Strong Or Weak Electrolyte.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Lead Acetate Strong Or Weak Electrolyte Weak acids are weak electrolytes, and most. Lead (ii) acetate is soluble in. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Strong electrolytes include the strong acids, strong bases, and salts. Salts are. Lead Acetate Strong Or Weak Electrolyte.
From www.numerade.com
SOLVED ' Classify each of the following solutes as a strong Lead Acetate Strong Or Weak Electrolyte Salts are often strong electrolytes, and strong acids are always strong electrolytes. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Lead (ii) acetate is soluble in. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Electrolytes are chemicals that break into ions. Lead Acetate Strong Or Weak Electrolyte.
From www.slideserve.com
PPT Chapter 13, (14) Mixtures and Aqueous Solutions PowerPoint Lead Acetate Strong Or Weak Electrolyte These chemicals completely dissociate into ions in aqueous solution. Weak acids are weak electrolytes, and most. Weak electrolytes less is more for these oddities which possess the remarkable ability to exhibit infinite equivalent conductivity at infinite dilution. Lead (ii) acetate is soluble in. Soluble salts tend to be strong electrolytes because they release charged ions into solution. Electrolytes are chemicals. Lead Acetate Strong Or Weak Electrolyte.