Chlorine Valence Core And Unpaired Electrons . Since chlorine is found in group 7a of the. — chlorine is in group viia (group 17), so it would have seven valence electrons. — carbon, which is located in group 4a, has 4 valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — there are two ways to find the number of valence electrons in chlorine. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. Calcium would have two valence electrons, since it is in group iia. So the valency of chlorine is 1.
from valenceelectrons.com
— carbon, which is located in group 4a, has 4 valence electrons. Calcium would have two valence electrons, since it is in group iia. — chlorine is in group viia (group 17), so it would have seven valence electrons. Since chlorine is found in group 7a of the. So the valency of chlorine is 1. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — there are two ways to find the number of valence electrons in chlorine. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those.
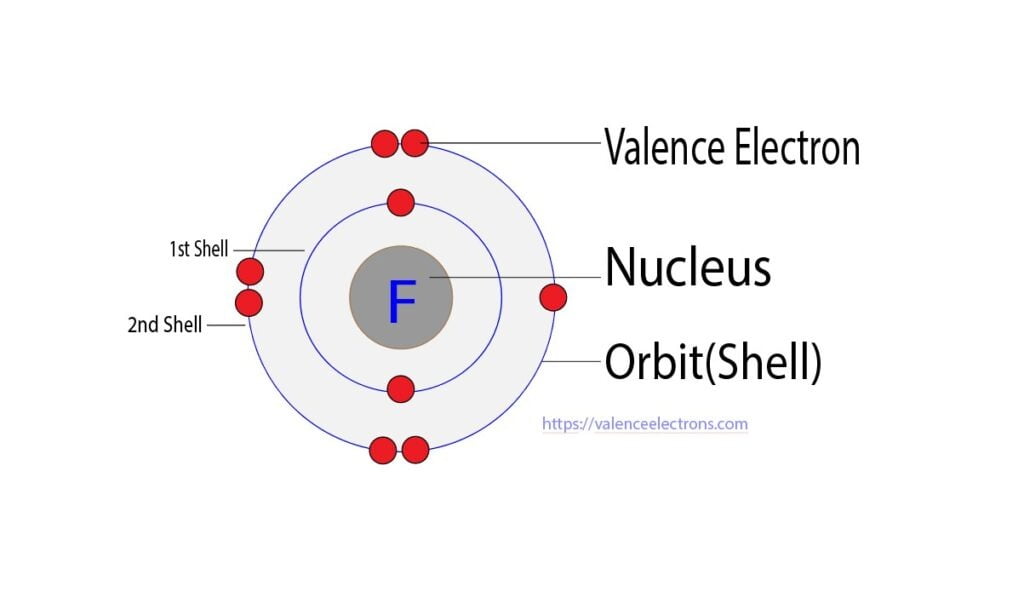
How Many Valence Electrons Does Fluorine (F) Have?
Chlorine Valence Core And Unpaired Electrons — carbon, which is located in group 4a, has 4 valence electrons. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — there are two ways to find the number of valence electrons in chlorine. So the valency of chlorine is 1. Since chlorine is found in group 7a of the. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Calcium would have two valence electrons, since it is in group iia. — chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — carbon, which is located in group 4a, has 4 valence electrons.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube Chlorine Valence Core And Unpaired Electrons — there are two ways to find the number of valence electrons in chlorine. — chlorine is in group viia (group 17), so it would have seven valence electrons. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — valence electrons occupy the outermost shell or highest energy level of. Chlorine Valence Core And Unpaired Electrons.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Valence Core And Unpaired Electrons — carbon, which is located in group 4a, has 4 valence electrons. So the valency of chlorine is 1. — there are two ways to find the number of valence electrons in chlorine. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. 93 rows — you. Chlorine Valence Core And Unpaired Electrons.
From www.youtube.com
Valence and core electrons YouTube Chlorine Valence Core And Unpaired Electrons — there are two ways to find the number of valence electrons in chlorine. — carbon, which is located in group 4a, has 4 valence electrons. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — this electron configuration shows that the last shell of the. Chlorine Valence Core And Unpaired Electrons.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram Chlorine Valence Core And Unpaired Electrons — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — there are two ways to find the number of valence electrons in chlorine. Since chlorine is found in. Chlorine Valence Core And Unpaired Electrons.
From schematicsarjairllixq7.z22.web.core.windows.net
Chlorine Electric Dot Diagram Chlorine Valence Core And Unpaired Electrons — carbon, which is located in group 4a, has 4 valence electrons. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — valence electrons occupy the outermost shell. Chlorine Valence Core And Unpaired Electrons.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Valence Core And Unpaired Electrons — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. So the valency of chlorine is 1. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — chlorine is in group viia (group 17), so it would have seven valence electrons. . Chlorine Valence Core And Unpaired Electrons.
From brokeasshome.com
Periodic Table Of Elements With Atomic Mass And Valency Chlorine Valence Core And Unpaired Electrons 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — there are two ways to find the number of valence electrons in chlorine. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). —. Chlorine Valence Core And Unpaired Electrons.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Chlorine Valence Core And Unpaired Electrons Calcium would have two valence electrons, since it is in group iia. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — chlorine is in group viia (group 17), so it would have seven valence electrons. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine. Chlorine Valence Core And Unpaired Electrons.
From giovhgnqp.blob.core.windows.net
Chlorine Atomic Number 17 Valence Electrons) at Mary Jennings blog Chlorine Valence Core And Unpaired Electrons — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. Calcium would have two valence electrons, since it is in group iia. Since chlorine is found in group 7a of. Chlorine Valence Core And Unpaired Electrons.
From giowhypxq.blob.core.windows.net
Chlorine Expanded Valence Shell at Bradley Maiorano blog Chlorine Valence Core And Unpaired Electrons Since chlorine is found in group 7a of the. So the valency of chlorine is 1. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. Calcium would have two valence electrons, since it is in group iia. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine. Chlorine Valence Core And Unpaired Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Chlorine Valence Core And Unpaired Electrons — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — chlorine is in group viia (group 17), so it would have seven valence electrons. . Chlorine Valence Core And Unpaired Electrons.
From giomjdqrz.blob.core.windows.net
How Many Electrons Does Chlorine Have at Matthew Rogers blog Chlorine Valence Core And Unpaired Electrons — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. So the valency of chlorine is 1. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. Calcium would have two valence electrons, since it is in group iia. — chlorine is in. Chlorine Valence Core And Unpaired Electrons.
From giomjdqrz.blob.core.windows.net
How Many Electrons Does Chlorine Have at Matthew Rogers blog Chlorine Valence Core And Unpaired Electrons — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — carbon, which is located in group 4a, has 4 valence electrons. Since chlorine is found in group 7a of the. Calcium would have two valence electrons, since it is in group iia. — there are two ways to find the number. Chlorine Valence Core And Unpaired Electrons.
From profliway.hubpages.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Chlorine Valence Core And Unpaired Electrons So the valency of chlorine is 1. — carbon, which is located in group 4a, has 4 valence electrons. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — chlorine is. Chlorine Valence Core And Unpaired Electrons.
From schematicfixgingles.z21.web.core.windows.net
Write The Orbital Box Diagram For Oxygen O Chlorine Valence Core And Unpaired Electrons — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. So the valency of chlorine is 1. — there are two ways to find the number of valence electrons in chlorine. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond. Chlorine Valence Core And Unpaired Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Valence Core And Unpaired Electrons 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Since chlorine is found in group 7a of the. — carbon, which is located in group 4a, has 4 valence electrons. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence. Chlorine Valence Core And Unpaired Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Fluorine (F) Have? Chlorine Valence Core And Unpaired Electrons Calcium would have two valence electrons, since it is in group iia. — chlorine is in group viia (group 17), so it would have seven valence electrons. — carbon, which is located in group 4a, has 4 valence electrons. Since chlorine is found in group 7a of the. — there are two ways to find the number. Chlorine Valence Core And Unpaired Electrons.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Valence Core And Unpaired Electrons So the valency of chlorine is 1. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. Since chlorine is found in group 7a of the. Calcium would have two valence electrons, since it is in group iia. — carbon, which is located in group 4a, has 4 valence. Chlorine Valence Core And Unpaired Electrons.
From circuitlistethiops123.z13.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Valence Core And Unpaired Electrons — carbon, which is located in group 4a, has 4 valence electrons. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — chlorine is in group viia (group. Chlorine Valence Core And Unpaired Electrons.
From giovhgnqp.blob.core.windows.net
Chlorine Atomic Number 17 Valence Electrons) at Mary Jennings blog Chlorine Valence Core And Unpaired Electrons — chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — there are two ways to find the number of valence electrons in chlorine. — this electron configuration shows. Chlorine Valence Core And Unpaired Electrons.
From valenceelectrons.com
How Many Valence Electrons Does SO2 (Sulfur Dioxide) Have? Chlorine Valence Core And Unpaired Electrons Since chlorine is found in group 7a of the. Calcium would have two valence electrons, since it is in group iia. So the valency of chlorine is 1. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — carbon, which is located in group 4a, has 4 valence. Chlorine Valence Core And Unpaired Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Chlorine Valence Core And Unpaired Electrons Since chlorine is found in group 7a of the. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — chlorine is in group viia (group 17), so it would have seven valence electrons. — carbon, which is located in group 4a, has 4 valence electrons. —. Chlorine Valence Core And Unpaired Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Valence Core And Unpaired Electrons Calcium would have two valence electrons, since it is in group iia. Since chlorine is found in group 7a of the. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — carbon, which is located in group 4a, has 4 valence electrons. So the valency. Chlorine Valence Core And Unpaired Electrons.
From hxeibtoad.blob.core.windows.net
Chloride Ion Of Valence Electrons at Autumn Norton blog Chlorine Valence Core And Unpaired Electrons — there are two ways to find the number of valence electrons in chlorine. Calcium would have two valence electrons, since it is in group iia. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — chlorine is in group viia (group 17), so it would have seven valence electrons. . Chlorine Valence Core And Unpaired Electrons.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Chlorine Valence Core And Unpaired Electrons Since chlorine is found in group 7a of the. — there are two ways to find the number of valence electrons in chlorine. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. Calcium would have two valence electrons, since it is in group iia. — carbon, which. Chlorine Valence Core And Unpaired Electrons.
From giomjdqrz.blob.core.windows.net
How Many Electrons Does Chlorine Have at Matthew Rogers blog Chlorine Valence Core And Unpaired Electrons 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Calcium would have two valence electrons, since it is in group iia. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. So the valency of chlorine is 1. —. Chlorine Valence Core And Unpaired Electrons.
From circuitlibsmoring.z21.web.core.windows.net
Orbital Diagram For Beryllium Chlorine Valence Core And Unpaired Electrons — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Since chlorine is found. Chlorine Valence Core And Unpaired Electrons.
From carmanchewchem.blogspot.com
Chemistry Atomic Structure Valency Chlorine Valence Core And Unpaired Electrons — there are two ways to find the number of valence electrons in chlorine. — chlorine is in group viia (group 17), so it would have seven valence electrons. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. — the #3s^2 3p^5# electrons are the outermost. Chlorine Valence Core And Unpaired Electrons.
From giowhypxq.blob.core.windows.net
Chlorine Expanded Valence Shell at Bradley Maiorano blog Chlorine Valence Core And Unpaired Electrons — carbon, which is located in group 4a, has 4 valence electrons. — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — valence electrons. Chlorine Valence Core And Unpaired Electrons.
From www.meritnation.com
What are valence and core electrons How many core electrons are there Chlorine Valence Core And Unpaired Electrons 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Since chlorine is found in group 7a of the. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — chlorine is in group viia (group 17), so it would. Chlorine Valence Core And Unpaired Electrons.
From dxofcoqqf.blob.core.windows.net
How Many Valence Electrons Are There In Chlorine at Candace Kelly blog Chlorine Valence Core And Unpaired Electrons Since chlorine is found in group 7a of the. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. — chlorine is in group viia (group 17), so it would have seven valence electrons. — there are two ways to find the number of valence electrons in chlorine. 93 rows —. Chlorine Valence Core And Unpaired Electrons.
From giowhypxq.blob.core.windows.net
Chlorine Expanded Valence Shell at Bradley Maiorano blog Chlorine Valence Core And Unpaired Electrons — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence electrons. Since chlorine is found in group 7a of the. — carbon, which is located in group 4a, has 4 valence electrons. So the valency of chlorine is 1. — there are two ways to find the number of valence electrons in chlorine.. Chlorine Valence Core And Unpaired Electrons.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Valence Core And Unpaired Electrons — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. So the valency of chlorine is 1. 93 rows — you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. — carbon, which is located in group 4a, has. Chlorine Valence Core And Unpaired Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Chlorine Valence Core And Unpaired Electrons — chlorine is in group viia (group 17), so it would have seven valence electrons. — valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those. Since chlorine is found in group 7a of the. — the #3s^2 3p^5# electrons are the outermost electrons, so chlorine has seven valence. Chlorine Valence Core And Unpaired Electrons.
From giowhypxq.blob.core.windows.net
Chlorine Expanded Valence Shell at Bradley Maiorano blog Chlorine Valence Core And Unpaired Electrons — this electron configuration shows that the last shell of the chlorine atom has an unpaired electron(3p z 1). — chlorine is in group viia (group 17), so it would have seven valence electrons. Since chlorine is found in group 7a of the. — carbon, which is located in group 4a, has 4 valence electrons. —. Chlorine Valence Core And Unpaired Electrons.