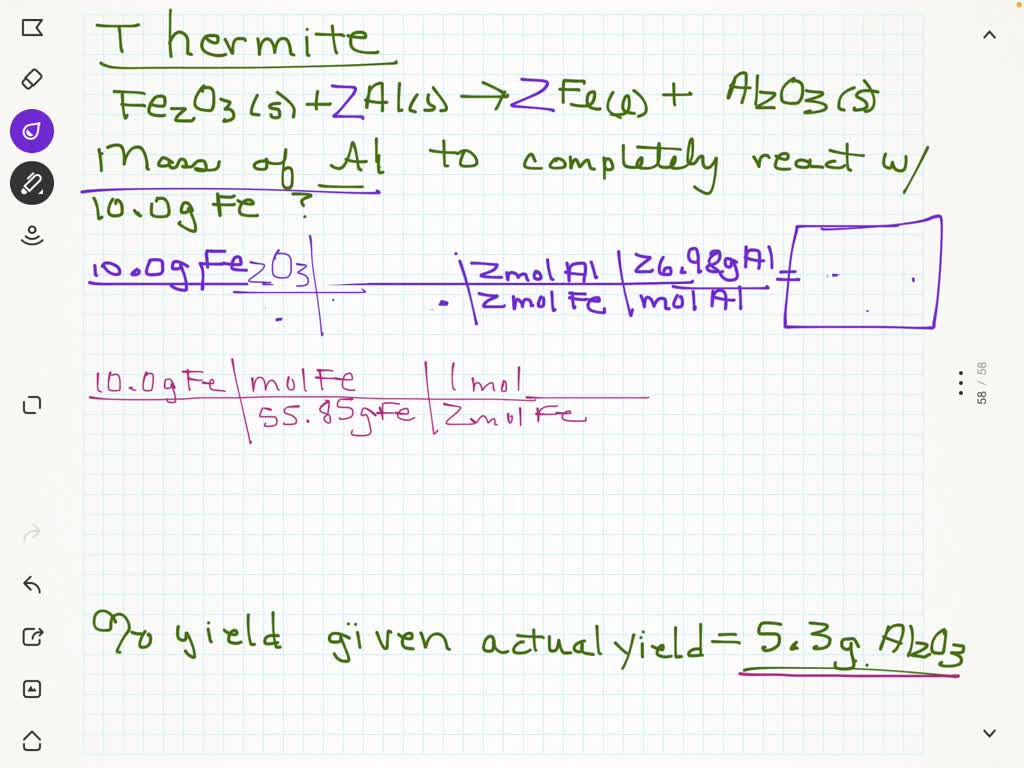
Iron Iii Oxide + Aluminum . This shows that aluminium is above iron in the reactivity series. Once underway, the reaction is highly exothermic,. This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. In the reaction between aluminum and iron (iii) oxide, this forms iron and. Aluminum replaces the metal in the oxide. This is because aluminum is more reactive than iron. Iron(iii) oxide + aluminium → aluminium oxide + iron. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium oxide (cr 2 o 3), or copper (ii) oxide may be used. This mixture of aluminum and iron oxide is called. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminum is almost always the metal that is oxidized. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of.
from www.numerade.com
This is because aluminum is more reactive than iron. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminum is almost always the metal that is oxidized. Once underway, the reaction is highly exothermic,. This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. In the reaction between aluminum and iron (iii) oxide, this forms iron and. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. This mixture of aluminum and iron oxide is called. Iron(iii) oxide + aluminium → aluminium oxide + iron.
SOLVED The thermite reaction occurs when iron(III) oxide reacts with
Iron Iii Oxide + Aluminum Once underway, the reaction is highly exothermic,. This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. Once underway, the reaction is highly exothermic,. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium oxide (cr 2 o 3), or copper (ii) oxide may be used. Aluminum is almost always the metal that is oxidized. Iron(iii) oxide + aluminium → aluminium oxide + iron. This shows that aluminium is above iron in the reactivity series. In the reaction between aluminum and iron (iii) oxide, this forms iron and. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. Aluminum replaces the metal in the oxide. This is because aluminum is more reactive than iron. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. This mixture of aluminum and iron oxide is called.
From www.slideserve.com
PPT Combustion analysis PowerPoint Presentation, free download ID Iron Iii Oxide + Aluminum Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium oxide (cr 2 o 3), or copper (ii) oxide may be used. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction. Iron Iii Oxide + Aluminum.
From www.numerade.com
SOLVED The chemical equation below represents the reaction between Iron Iii Oxide + Aluminum A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This shows that aluminium is above iron in the reactivity series. Aluminum replaces the metal in the oxide. This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. Although black. Iron Iii Oxide + Aluminum.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron Iii Oxide + Aluminum A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This mixture of aluminum and iron oxide is called. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive. Iron Iii Oxide + Aluminum.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide + Aluminum This mixture of aluminum and iron oxide is called. This is because aluminum is more reactive than iron. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. Aluminum replaces the metal in the oxide. This shows that aluminium is above iron in the. Iron Iii Oxide + Aluminum.
From www.numerade.com
SOLVEDIn the thermite reaction, iron(III) oxide is reduced by aluminum Iron Iii Oxide + Aluminum Iron(iii) oxide + aluminium → aluminium oxide + iron. This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al +. Iron Iii Oxide + Aluminum.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron Iii Oxide + Aluminum This shows that aluminium is above iron in the reactivity series. Iron(iii) oxide + aluminium → aluminium oxide + iron. Aluminum replaces the metal in the oxide. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno. Iron Iii Oxide + Aluminum.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide + Aluminum A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This mixture of aluminum and iron oxide is called. This shows that aluminium is above iron in the reactivity series. Aluminum is almost always the metal that is oxidized. Fe2o3 + al = fe + al2o3. Iron Iii Oxide + Aluminum.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide + Aluminum This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. In the reaction between aluminum and iron (iii) oxide, this forms iron and. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces. Iron Iii Oxide + Aluminum.
From www.youtube.com
Thermit reaction, iron (III) oxide reacts with aluminium andgives Iron Iii Oxide + Aluminum This is because aluminum is more reactive than iron. Once underway, the reaction is highly exothermic,. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium oxide (cr 2 o 3), or copper (ii) oxide. Iron Iii Oxide + Aluminum.
From www.youtube.com
Thermit reaction iron (III) oxide reacts with aluminium and gives Iron Iii Oxide + Aluminum This shows that aluminium is above iron in the reactivity series. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. This is because aluminum is more reactive than iron. Once underway, the reaction is. Iron Iii Oxide + Aluminum.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide + Aluminum Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. This is because aluminum is more reactive than iron. Aluminum replaces the metal in the oxide. Aluminum is almost always the metal that is oxidized. This demonstration shows the highly exothermic reaction between aluminium. Iron Iii Oxide + Aluminum.
From www.numerade.com
SOLVED The thermite reaction occurs when iron(III) oxide reacts with Iron Iii Oxide + Aluminum This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. Iron(iii) oxide + aluminium → aluminium oxide + iron.. Iron Iii Oxide + Aluminum.
From testbook.com
Oxide Learn Structure, Preparation, Properties & Uses Iron Iii Oxide + Aluminum This mixture of aluminum and iron oxide is called. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium oxide (cr 2 o 3), or copper (ii) oxide may be used. Aluminium + iron(iii) oxide. Iron Iii Oxide + Aluminum.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron Iii Oxide + Aluminum This is because aluminum is more reactive than iron. Once underway, the reaction is highly exothermic,. Aluminum is almost always the metal that is oxidized. This shows that aluminium is above iron in the reactivity series. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium. Iron Iii Oxide + Aluminum.
From www.numerade.com
SOLVED Question 2 10 pts In a redox reaction, solid iron (III) oxide Iron Iii Oxide + Aluminum In the reaction between aluminum and iron (iii) oxide, this forms iron and. This is because aluminum is more reactive than iron. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This mixture of aluminum and iron oxide is called. This demonstration shows the highly. Iron Iii Oxide + Aluminum.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Iron Iii Oxide + Aluminum This is because aluminum is more reactive than iron. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This. Iron Iii Oxide + Aluminum.
From www.alamy.com
A thermite reaction (iron (III) oxide & aluminium powder) in a UK Iron Iii Oxide + Aluminum Once underway, the reaction is highly exothermic,. In the reaction between aluminum and iron (iii) oxide, this forms iron and. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminum replaces the metal in the oxide. This shows that aluminium is above iron in the. Iron Iii Oxide + Aluminum.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron Iii Oxide + Aluminum Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii). Iron Iii Oxide + Aluminum.
From edu.rsc.org
The thermite reaction between aluminium and iron(III) oxide Iron Iii Oxide + Aluminum This is because aluminum is more reactive than iron. Aluminum is almost always the metal that is oxidized. Aluminum replaces the metal in the oxide. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium. Iron Iii Oxide + Aluminum.
From www.youtube.com
Al+Fe2O3=Al2O3+Fe Balanced EquationAluminum+Iron(III) oxide+Iron Iron Iii Oxide + Aluminum Iron(iii) oxide + aluminium → aluminium oxide + iron. Aluminum replaces the metal in the oxide. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. This is because aluminum is more reactive than iron.. Iron Iii Oxide + Aluminum.
From www.chegg.com
Solved The reaction between aluminum and iron(III) oxide Iron Iii Oxide + Aluminum Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. Once underway, the reaction is highly exothermic,. Aluminum is almost always the metal that is oxidized. This is because aluminum is more reactive than iron. This mixture of aluminum and iron oxide is called.. Iron Iii Oxide + Aluminum.
From www.chemkits.eu
Iron(III) oxide, 99.0+, 1309371 Iron Iii Oxide + Aluminum Once underway, the reaction is highly exothermic,. This is because aluminum is more reactive than iron. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe +. Iron Iii Oxide + Aluminum.
From www.researchgate.net
Iron precursor hydrated iron (III) oxide (FeO(OH)) placed on aluminum Iron Iii Oxide + Aluminum This mixture of aluminum and iron oxide is called. Once underway, the reaction is highly exothermic,. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium oxide (cr 2 o 3), or copper (ii) oxide. Iron Iii Oxide + Aluminum.
From www.ceramic-glazes.com
IRON OXIDE Iron (III) Oxide Minium Pigments and Stains Iron Iii Oxide + Aluminum This mixture of aluminum and iron oxide is called. This is because aluminum is more reactive than iron. This shows that aluminium is above iron in the reactivity series. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminum replaces the metal in the oxide.. Iron Iii Oxide + Aluminum.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide + Aluminum This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. This shows that aluminium is above iron in the reactivity series. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminium + iron(iii) oxide → iron + aluminium oxide. Iron Iii Oxide + Aluminum.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide + Aluminum Aluminum is almost always the metal that is oxidized. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. This demonstration shows the highly exothermic reaction between aluminium and iron(iii) oxide that produces molten iron. Aluminium + iron(iii) oxide → iron + aluminium oxide. Iron Iii Oxide + Aluminum.
From brainly.in
write the skeletal equation and balance the following 1) iron (3) oxide Iron Iii Oxide + Aluminum Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii). Iron Iii Oxide + Aluminum.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron Iii Oxide + Aluminum Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. This shows that aluminium is above iron in the reactivity series. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide. Iron Iii Oxide + Aluminum.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron Iii Oxide + Aluminum Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii). Iron Iii Oxide + Aluminum.
From www.numerade.com
SOLVED The highly exothermic thermite reaction, in which aluminum Iron Iii Oxide + Aluminum Aluminum replaces the metal in the oxide. Iron(iii) oxide + aluminium → aluminium oxide + iron. This shows that aluminium is above iron in the reactivity series. This is because aluminum is more reactive than iron. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium. Iron Iii Oxide + Aluminum.
From wizedu.com
If 87.5g of molten iron (III) oxide reacts with 25.7g of aluminum Iron Iii Oxide + Aluminum Once underway, the reaction is highly exothermic,. This mixture of aluminum and iron oxide is called. Aluminum is almost always the metal that is oxidized. This is because aluminum is more reactive than iron. This shows that aluminium is above iron in the reactivity series. Aluminum replaces the metal in the oxide. A thermite reaction or process is an exothermic. Iron Iii Oxide + Aluminum.
From www.bartleby.com
Answered The thermite reaction occurs when… bartleby Iron Iii Oxide + Aluminum Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii). Iron Iii Oxide + Aluminum.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Iii Oxide + Aluminum Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii) oxide (fe 2 o 3), manganese oxide (mno 2), chromium oxide (cr 2 o 3), or copper (ii) oxide may be used. Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction. Iron Iii Oxide + Aluminum.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide + Aluminum This shows that aluminium is above iron in the reactivity series. This is because aluminum is more reactive than iron. Aluminium + iron(iii) oxide → iron + aluminium oxide 2al + fe 2 o 3 → 2fe + al 2 o 3 as aluminium is more reactive than iron, it displaces iron from iron(iii) oxide. A thermite reaction or process. Iron Iii Oxide + Aluminum.
From noahchemicals.com
3 Manufacturing Applications for Iron (III) Oxide Noah Chemicals Iron Iii Oxide + Aluminum Fe2o3 + al = fe + al2o3 is a single displacement (substitution) reaction where one mole of hematite [fe 2 o 3] and two moles of. This mixture of aluminum and iron oxide is called. Although black or blue iron oxide (fe 3 o 4) is most often used as an oxidizing agent in the thermite reaction, red iron (iii). Iron Iii Oxide + Aluminum.