Buffer Solution List . These buffer solutions are used to regulate the ph of a solution. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution can resist ph change because of an equilibrium. In addition to the tables below,. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. It is able to neutralize small amounts of added acid or base, thus. They are resistant to ph changes. What is a buffer solution? Definition a buffer solution is one which resists changes in ph. What is a buffer solution? A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The following tables can help you navigate preparation of many common buffer solutions by ph and pka.
from www.slideserve.com
What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. In addition to the tables below,. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The following tables can help you navigate preparation of many common buffer solutions by ph and pka. These buffer solutions are used to regulate the ph of a solution. What is a buffer solution? A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. It is able to neutralize small amounts of added acid or base, thus.
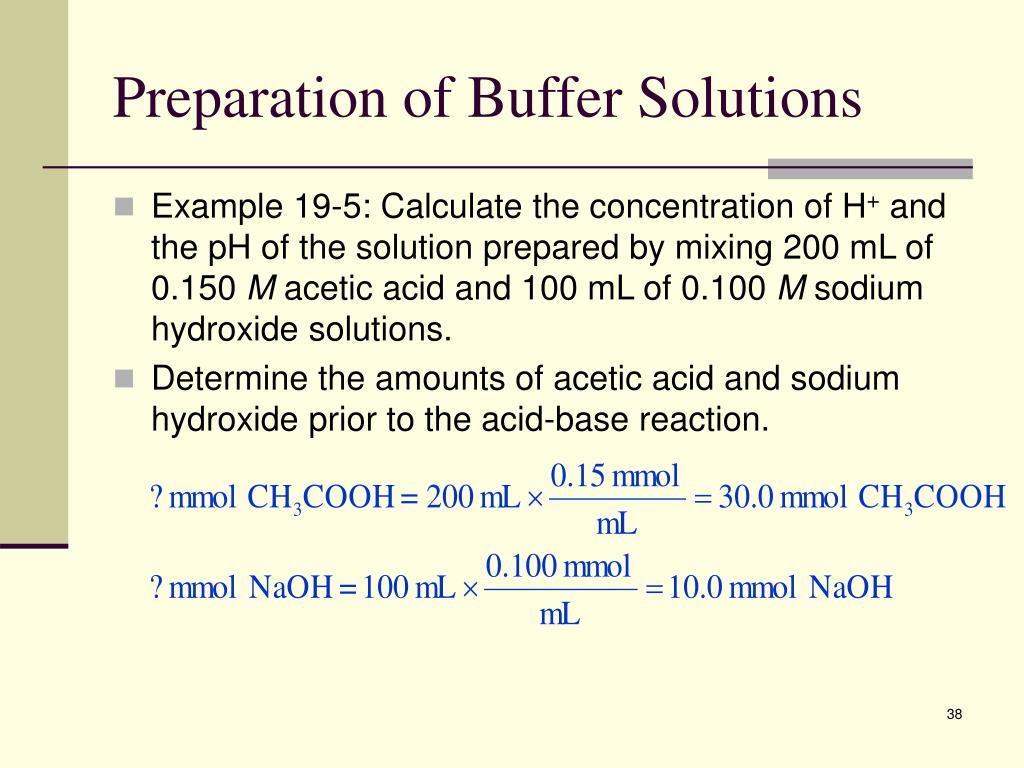
PPT Chapter 17a PowerPoint Presentation, free download ID4339402
Buffer Solution List Definition a buffer solution is one which resists changes in ph. These buffer solutions are used to regulate the ph of a solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. It is able to neutralize small amounts of added acid or base, thus. This page describes simple acidic and alkaline buffer solutions and explains how they work. They are resistant to ph changes. What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution can resist ph change because of an equilibrium. In addition to the tables below,. The following tables can help you navigate preparation of many common buffer solutions by ph and pka. What is a buffer solution? Definition a buffer solution is one which resists changes in ph. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
From www.youtube.com
Buffer Solutions YouTube Buffer Solution List Definition a buffer solution is one which resists changes in ph. This page describes simple acidic and alkaline buffer solutions and explains how they work. It is able to neutralize small amounts of added acid or base, thus. These buffer solutions are used to regulate the ph of a solution. What is a buffer solution? A buffer solution is one. Buffer Solution List.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 Buffer Solution List A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. In addition to the tables below,. What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that can resist ph. Buffer Solution List.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Solution List These buffer solutions are used to regulate the ph of a solution. The following tables can help you navigate preparation of many common buffer solutions by ph and pka. What is a buffer solution? They are resistant to ph changes. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt.. Buffer Solution List.
From www.myronl.com
Standard Solutions & Buffers Myron L® Company Buffer Solution List This page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer solution can resist ph change because of an equilibrium. Definition a buffer solution is one which resists changes in. Buffer Solution List.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk Buffer Solution List The following tables can help you navigate preparation of many common buffer solutions by ph and pka. It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. They are resistant to ph changes. What is a buffer. Buffer Solution List.
From www.biophlox.com
Buy PH Buffer Solution get price for lab equipment Buffer Solution List These buffer solutions are used to regulate the ph of a solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. The following tables can help you navigate preparation. Buffer Solution List.
From general.chemistrysteps.com
Buffer Solutions Chemistry Steps Buffer Solution List A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. These buffer solutions are used to regulate the ph of a solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer is a solution that can resist. Buffer Solution List.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Buffer Solution List A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Definition a buffer solution is one which resists changes in ph. In addition to the tables below,. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a combination of strong acid and. Buffer Solution List.
From www.pinterest.com
Pin on Download Buffer Solution List A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Definition a buffer solution is one which resists changes in ph. These buffer solutions are used to regulate the ph of a solution. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution. Buffer Solution List.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk Buffer Solution List A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. They are resistant to ph changes. This page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? The following tables can help you navigate preparation of many common buffer solutions by ph. Buffer Solution List.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution List They are resistant to ph changes. Definition a buffer solution is one which resists changes in ph. These buffer solutions are used to regulate the ph of a solution. A buffer solution can resist ph change because of an equilibrium. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are.. Buffer Solution List.
From facts.net
13 Enigmatic Facts About Buffer Capacity Buffer Solution List Definition a buffer solution is one which resists changes in ph. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. This page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? A buffer solution is one which resists changes in ph. Buffer Solution List.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Buffer Solution List Definition a buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In addition to the tables below,. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. It is able to. Buffer Solution List.
From www.numerade.com
Question 1 (2 points) Which acids from the list below cannot be used to Buffer Solution List A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. What is a buffer solution? A buffer. Buffer Solution List.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution List This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. They are resistant to ph changes. These buffer solutions are used to regulate the ph of a solution. What is a buffer solution? In addition to. Buffer Solution List.
From studylib.net
The SOLUTION for All of Your Buffer Needs Buffer Solution List A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. They are resistant to ph changes. A buffer solution can resist ph change because of an equilibrium. These buffer solutions are. Buffer Solution List.
From www.indiamart.com
Ww 00654xx Oakton pH Buffer Solution 500 mL, Grade Standard Buffer Solution List It is able to neutralize small amounts of added acid or base, thus. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. These buffer solutions are used to regulate the ph of a solution. Definition a buffer solution is one which resists changes in ph. The following tables can. Buffer Solution List.
From www.semanticscholar.org
Table 1 from Buffer solutions in drug formulation and processing How Buffer Solution List The following tables can help you navigate preparation of many common buffer solutions by ph and pka. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Definition a buffer solution is one which resists changes in ph. What is a buffer solution? In addition to the tables below,. It. Buffer Solution List.
From ssh-gulf.com
Buffer Solution pH 10 Scientific Supply House Buffer Solution List This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. In addition to the tables below,. The following tables can help you navigate preparation of many common buffer solutions by ph and pka. A buffer solution. Buffer Solution List.
From parkesscientific.com
Buffer Solution, Item 1645, pH 10.00, (Color Coded Blue), NIST Buffer Solution List These buffer solutions are used to regulate the ph of a solution. They are resistant to ph changes. It is able to neutralize small amounts of added acid or base, thus. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The following tables can help you navigate preparation of. Buffer Solution List.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution List A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. These buffer solutions are used to regulate the ph of a solution. They are resistant to ph changes. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a. Buffer Solution List.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Buffer Solution List They are resistant to ph changes. Definition a buffer solution is one which resists changes in ph. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. A buffer solution can resist ph change because of an equilibrium. It is able to neutralize small amounts of added acid or base,. Buffer Solution List.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution List This page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer is a combination of strong. Buffer Solution List.
From preclaboratories.com
pH and Buffers How Buffer Solutions Maintain pH Precision Laboratories Buffer Solution List What is a buffer solution? They are resistant to ph changes. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. The following tables can help you navigate preparation of many common buffer solutions by ph and pka. This page describes simple acidic and alkaline buffer solutions and explains how. Buffer Solution List.
From www.lookfordiagnosis.com
Buffers Buffer Solution List A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. What is a buffer solution? What is a buffer solution? These buffer solutions are used to regulate the ph of a solution. In addition to the tables below,. They are resistant to ph changes. A buffer is a solution that. Buffer Solution List.
From www.sliderbase.com
Buffers Presentation Chemistry Buffer Solution List Definition a buffer solution is one which resists changes in ph. A buffer solution can resist ph change because of an equilibrium. They are resistant to ph changes. The following tables can help you navigate preparation of many common buffer solutions by ph and pka. What is a buffer solution? It is able to neutralize small amounts of added acid. Buffer Solution List.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Buffer Solution List A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small amounts of added acid or base, thus. What is a buffer solution? A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. They are. Buffer Solution List.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses Buffer Solution List These buffer solutions are used to regulate the ph of a solution. It is able to neutralize small amounts of added acid or base, thus. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Definition a buffer solution is one which resists changes in ph. The following tables can. Buffer Solution List.
From japaneseclass.jp
Images of バッファ JapaneseClass.jp Buffer Solution List It is able to neutralize small amounts of added acid or base, thus. What is a buffer solution? In addition to the tables below,. Definition a buffer solution is one which resists changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. These buffer solutions are used to. Buffer Solution List.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Buffer Solution List A buffer solution can resist ph change because of an equilibrium. These buffer solutions are used to regulate the ph of a solution. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The following tables can help you navigate preparation of many common buffer solutions by ph and pka.. Buffer Solution List.
From www.pdfprof.com
PDF examples of buffer solutions PDF Télécharger Download Buffer Solution List A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? Definition a buffer solution is one which resists changes in ph. They are resistant to ph changes. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate. Buffer Solution List.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Buffer Solution List A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. These buffer solutions are used to regulate the ph of a solution. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. In addition to the tables below,. A buffer solution. Buffer Solution List.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog Buffer Solution List The following tables can help you navigate preparation of many common buffer solutions by ph and pka. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate. Buffer Solution List.
From www.chegg.com
A buffer solution is prepared by mixing 43.1 mL of Buffer Solution List Definition a buffer solution is one which resists changes in ph. These buffer solutions are used to regulate the ph of a solution. The following tables can help you navigate preparation of many common buffer solutions by ph and pka. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt.. Buffer Solution List.
From www.studyxapp.com
part 1 preparation of a buffer solution solution 1 1000 ml assigned ph Buffer Solution List In addition to the tables below,. A buffer is a combination of strong acid and its basic salt or a strong base and its acidic salt. These buffer solutions are used to regulate the ph of a solution. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. Definition a. Buffer Solution List.