Lead Number Of Electrons Lost . Hydrogen is an exception, as it will usually. When a lead atom loses two electrons it exhibits a +2 charge. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. These elements lie to the left in a period or near. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. First, we should understand that lead(i) is pb^(1+). In equation 4.4.3, for example, the total number of electrons lost. Many metallic elements have relatively low ionization potentials and lose electrons easily. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. 1, 2 and 3, the number of electrons lost is the same as the group number.
from www.numerade.com
These elements lie to the left in a period or near. First, we should understand that lead(i) is pb^(1+). 1, 2 and 3, the number of electrons lost is the same as the group number. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. Many metallic elements have relatively low ionization potentials and lose electrons easily. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. In equation 4.4.3, for example, the total number of electrons lost.
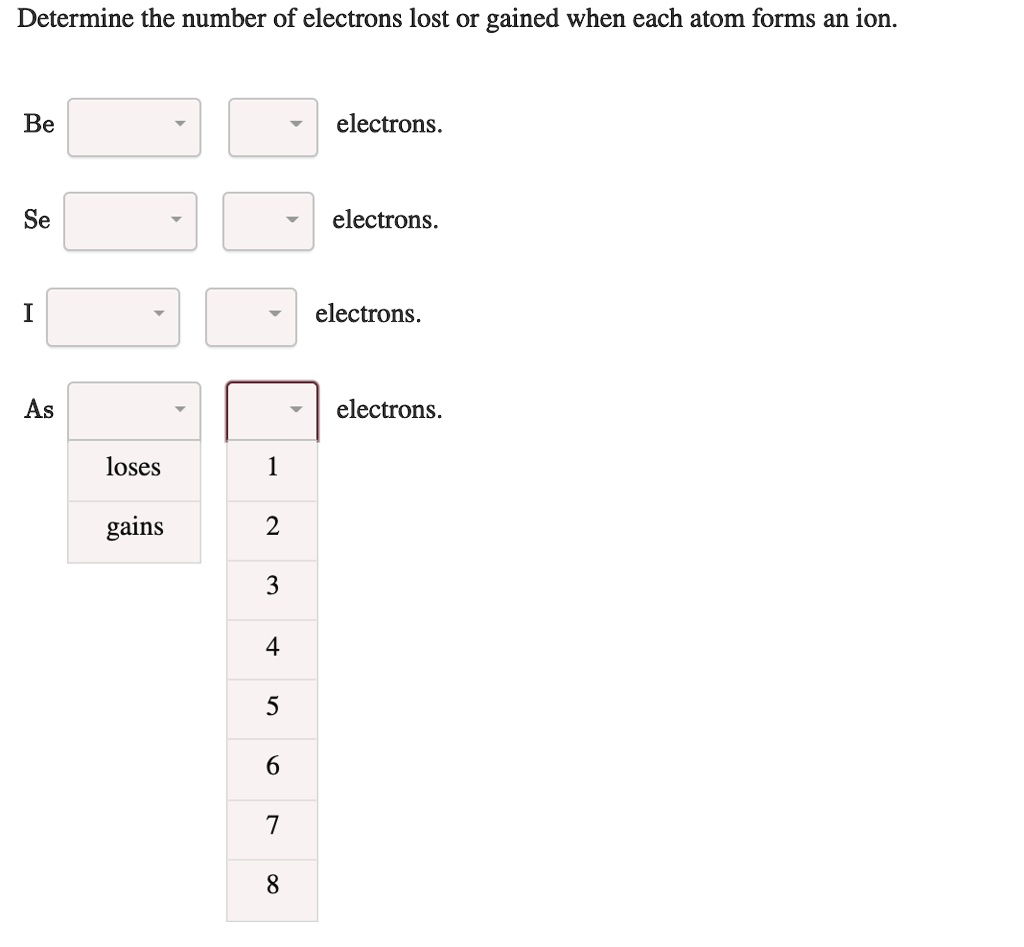
SOLVED Determine the number of electrons lost O gained when each atom
Lead Number Of Electrons Lost These elements lie to the left in a period or near. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. In equation 4.4.3, for example, the total number of electrons lost. 1, 2 and 3, the number of electrons lost is the same as the group number. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. When a lead atom loses two electrons it exhibits a +2 charge. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. These elements lie to the left in a period or near. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Many metallic elements have relatively low ionization potentials and lose electrons easily. Hydrogen is an exception, as it will usually. First, we should understand that lead(i) is pb^(1+).
From byjus.com
A sphere of lead of mass 10 g has net charge 2.5 x 10 9 O(a) Find the Lead Number Of Electrons Lost When a lead atom loses two electrons it exhibits a +2 charge. Hydrogen is an exception, as it will usually. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. 1, 2 and 3, the number of electrons lost is the same as the group number. Now,. Lead Number Of Electrons Lost.
From www.numerade.com
⏩SOLVEDThe number of electrons lost in the following change is… Numerade Lead Number Of Electrons Lost Many metallic elements have relatively low ionization potentials and lose electrons easily. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. First, we should understand that lead(i) is pb^(1+). In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to. Lead Number Of Electrons Lost.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead Number Of Electrons Lost In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. In any redox. Lead Number Of Electrons Lost.
From iperiodictable.com
How To Find an Valence Lead Electron Configuration (Pb) Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. Hydrogen is an exception, as it will usually. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. Lead Number Of Electrons Lost.
From www.slideserve.com
PPT VIII. OxidationReduction PowerPoint Presentation, free download Lead Number Of Electrons Lost So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. These elements lie to the left in a period or near. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Hydrogen is an exception, as it will. Lead Number Of Electrons Lost.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. When a lead atom loses two electrons it exhibits a +2 charge. These elements lie to the left in a period or near. In equation 4.4.3, for example, the. Lead Number Of Electrons Lost.
From www.numerade.com
SOLVED Determine the number of electrons lost O gained when each atom Lead Number Of Electrons Lost Now, from this, we can find the number of unpaired electrons in a lead(i) ion. First, we should understand that lead(i) is pb^(1+). These elements lie to the left in a period or near. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. In equation 4.4.3, for example,. Lead Number Of Electrons Lost.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. Hydrogen is an exception, as it will usually. So, these two electrons will be lost from. Lead Number Of Electrons Lost.
From www.researchgate.net
Average number of electrons lost in a Photosystem I (PSI) crystal vs Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. When a lead atom loses two electrons it exhibits a +2 charge. In any redox reaction, the total number of electrons lost must equal the total of electrons gained. Lead Number Of Electrons Lost.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Number Of Electrons Lost In equation 4.4.3, for example, the total number of electrons lost. When a lead atom loses two electrons it exhibits a +2 charge. Hydrogen is an exception, as it will usually. First, we should understand that lead(i) is pb^(1+). So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital.. Lead Number Of Electrons Lost.
From quizzma.com
When a honeybee flies through the air it develops a charge of +20pC Lead Number Of Electrons Lost In equation 4.4.3, for example, the total number of electrons lost. 1, 2 and 3, the number of electrons lost is the same as the group number. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Now, from this, we can find the number of unpaired. Lead Number Of Electrons Lost.
From www.numerade.com
SOLVED Determine the number of electrons lost Or gained when each atom Lead Number Of Electrons Lost Many metallic elements have relatively low ionization potentials and lose electrons easily. In equation 4.4.3, for example, the total number of electrons lost. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. Hydrogen is an exception, as it will usually. These elements lie to the left in a period or near. Atoms tend to. Lead Number Of Electrons Lost.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Number Of Electrons Lost These elements lie to the left in a period or near. Hydrogen is an exception, as it will usually. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. Many metallic elements have relatively low ionization potentials and lose electrons easily. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire. Lead Number Of Electrons Lost.
From www.numerade.com
SOLVED Indicate the number of electrons lost or gained when each of Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. These elements lie to the left in a period or near. 1, 2 and 3, the number of electrons lost is the same as the group number. Many metallic elements have relatively low ionization potentials and lose. Lead Number Of Electrons Lost.
From www.vrogue.co
Calculate The Number Of Electrons Lost Or Gained Duri vrogue.co Lead Number Of Electrons Lost These elements lie to the left in a period or near. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. Hydrogen is an exception, as it will usually. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Atoms tend to lose, gain,. Lead Number Of Electrons Lost.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Number Of Electrons Lost Now, from this, we can find the number of unpaired electrons in a lead(i) ion. First, we should understand that lead(i) is pb^(1+). Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. 1, 2 and 3, the number of electrons lost is the same as the. Lead Number Of Electrons Lost.
From www.numerade.com
SOLVED Determine the number of electrons lost or gained when each atom Lead Number Of Electrons Lost So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Hydrogen is an exception, as it will usually. In any redox reaction, the total number of. Lead Number Of Electrons Lost.
From www.youtube.com
Number of Electrons in Lithium (Li) YouTube Lead Number Of Electrons Lost These elements lie to the left in a period or near. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. Hydrogen is an exception, as it will usually. In equation 4.4.3, for example, the total number of electrons lost. In general, metals will lose electrons to become a. Lead Number Of Electrons Lost.
From opentextbc.ca
6.4 Electronic Structure of Atoms (Electron Configurations) Chemistry Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. In equation 4.4.3, for example, the total number. Lead Number Of Electrons Lost.
From www.chegg.com
Solved Determine the number of electrons lost or gained when Lead Number Of Electrons Lost First, we should understand that lead(i) is pb^(1+). Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital.. Lead Number Of Electrons Lost.
From brennan-bogspotberry.blogspot.com
Does Selenium Gain or Lose Electrons Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. Hydrogen is an exception, as it will usually. Many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a period. Lead Number Of Electrons Lost.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. When a lead atom loses two electrons it exhibits a +2 charge. In any redox reaction, the total number of electrons lost must equal the total of electrons gained. Lead Number Of Electrons Lost.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead Number Of Electrons Lost 1, 2 and 3, the number of electrons lost is the same as the group number. In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Lead Number Of Electrons Lost.
From slideplayer.com
Oxidation Numbers. ppt download Lead Number Of Electrons Lost These elements lie to the left in a period or near. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for. Lead Number Of Electrons Lost.
From byjus.com
Calculate the no. of electrons in 100 gms of CO2 Lead Number Of Electrons Lost 1, 2 and 3, the number of electrons lost is the same as the group number. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Hydrogen is an exception, as it. Lead Number Of Electrons Lost.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Lead Number Of Electrons Lost When a lead atom loses two electrons it exhibits a +2 charge. First, we should understand that lead(i) is pb^(1+). So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. 1, 2 and 3, the number of electrons lost is the same as the group number. These elements lie. Lead Number Of Electrons Lost.
From wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons Lead Number Of Electrons Lost In equation 4.4.3, for example, the total number of electrons lost. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e., usually eight valence electrons for the main group. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of. Lead Number Of Electrons Lost.
From www.slideshare.net
Understanding The Chemistry Of Atoms to Ions Lead Number Of Electrons Lost When a lead atom loses two electrons it exhibits a +2 charge. First, we should understand that lead(i) is pb^(1+). These elements lie to the left in a period or near. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Many metallic elements have relatively low ionization potentials. Lead Number Of Electrons Lost.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Lead Number Of Electrons Lost So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. Hydrogen is an exception, as it will usually. Now, from this, we can find the number of unpaired. Lead Number Of Electrons Lost.
From www.slideserve.com
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID Lead Number Of Electrons Lost In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of. Lead Number Of Electrons Lost.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Lead Number Of Electrons Lost So, these two electrons will be lost from the last shell, which means electrons will be lost from the 6p orbital. When a lead atom loses two electrons it exhibits a +2 charge. Many metallic elements have relatively low ionization potentials and lose electrons easily. First, we should understand that lead(i) is pb^(1+). Now, from this, we can find the. Lead Number Of Electrons Lost.
From www.researchgate.net
3. Fractional energy loss per radiation length in lead as a function Lead Number Of Electrons Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas,. Lead Number Of Electrons Lost.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes Lead Number Of Electrons Lost In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Many metallic elements have relatively low ionization potentials and lose electrons easily. Atoms tend to lose,. Lead Number Of Electrons Lost.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Lead Number Of Electrons Lost In equation 4.4.3, for example, the total number of electrons lost. Many metallic elements have relatively low ionization potentials and lose electrons easily. First, we should understand that lead(i) is pb^(1+). Now, from this, we can find the number of unpaired electrons in a lead(i) ion. When a lead atom loses two electrons it exhibits a +2 charge. In any. Lead Number Of Electrons Lost.
From www.bartleby.com
Answered Lead is normally obtained from PbS… bartleby Lead Number Of Electrons Lost First, we should understand that lead(i) is pb^(1+). When a lead atom loses two electrons it exhibits a +2 charge. Now, from this, we can find the number of unpaired electrons in a lead(i) ion. In any redox reaction, the total number of electrons lost must equal the total of electrons gained to preserve electrical neutrality. In equation 4.4.3, for. Lead Number Of Electrons Lost.