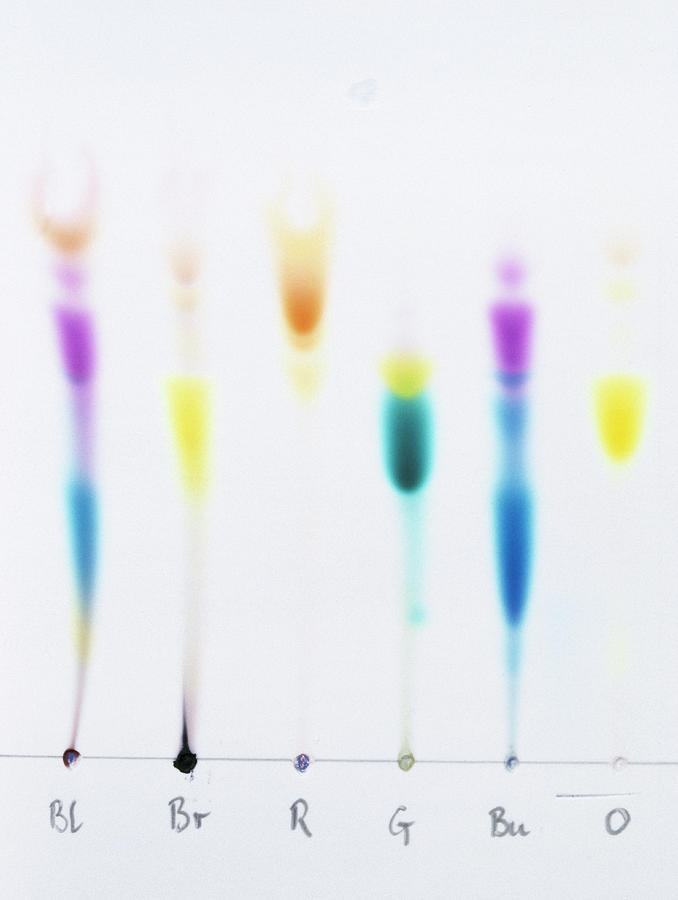In Paper Chromatography What Do The Different Spots Represent . R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. if the spots can be seen, outline them with a pencil. each spot represents a different component found in the original mixture. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. If the spots are not obvious, the. in paper chromatography, the stationary phase is a very uniform absorbent paper. Each component moves up the paper at a different speed, depending on its relative. the r f value for each spot should be calculated. a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. The mobile phase is a suitable liquid solvent (typically, water) or. Chromatography is used to separate mixtures of. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. Sometimes the color will fade over time, so the pencil outline is important.
from sciencephotogallery.com
each spot represents a different component found in the original mixture. the r f value for each spot should be calculated. If the spots are not obvious, the. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. if the spots can be seen, outline them with a pencil. Sometimes the color will fade over time, so the pencil outline is important. The mobile phase is a suitable liquid solvent (typically, water) or. in paper chromatography, the stationary phase is a very uniform absorbent paper. Each component moves up the paper at a different speed, depending on its relative.
Paper Chromatography by Andrew Lambert Photography
In Paper Chromatography What Do The Different Spots Represent once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. Sometimes the color will fade over time, so the pencil outline is important. If the spots are not obvious, the. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. the r f value for each spot should be calculated. Chromatography is used to separate mixtures of. in paper chromatography, the stationary phase is a very uniform absorbent paper. a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. if the spots can be seen, outline them with a pencil. each spot represents a different component found in the original mixture. The mobile phase is a suitable liquid solvent (typically, water) or. Each component moves up the paper at a different speed, depending on its relative.
From exoirwzyi.blob.core.windows.net
What Solvents Are Used In Paper Chromatography at Eleanora Hiott blog In Paper Chromatography What Do The Different Spots Represent If the spots are not obvious, the. Chromatography is used to separate mixtures of. Each component moves up the paper at a different speed, depending on its relative. if the spots can be seen, outline them with a pencil. The mobile phase is a suitable liquid solvent (typically, water) or. R f stands for ratio of fronts, or retardation. In Paper Chromatography What Do The Different Spots Represent.
From www.pinterest.com
What Is Paper Chromatography and How Does it Work? High school In Paper Chromatography What Do The Different Spots Represent once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. Each component moves up the paper. In Paper Chromatography What Do The Different Spots Represent.
From www.alamy.com
Paper chromatography. Coloured streaks left by different colour inks in In Paper Chromatography What Do The Different Spots Represent a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. If the spots are not obvious, the. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound. In Paper Chromatography What Do The Different Spots Represent.
From thechemistrynotes.com
Paper Chromatography In Paper Chromatography What Do The Different Spots Represent if the spots can be seen, outline them with a pencil. Each component moves up the paper at a different speed, depending on its relative. each spot represents a different component found in the original mixture. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. Sometimes the. In Paper Chromatography What Do The Different Spots Represent.
From hubpages.com
What Is Paper Chromatography Principle, Types, & Uses Owlcation In Paper Chromatography What Do The Different Spots Represent in paper chromatography, the stationary phase is a very uniform absorbent paper. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. Each component moves up the paper at a different speed, depending. In Paper Chromatography What Do The Different Spots Represent.
From animalia-life.club
Paper Chromatography Diagram In Paper Chromatography What Do The Different Spots Represent Chromatography is used to separate mixtures of. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. if the spots can be seen, outline them with a pencil. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. a pure substance produces. In Paper Chromatography What Do The Different Spots Represent.
From www.biochemden.com
What Is Paper Chromatography? Principle And Procedure In Paper Chromatography What Do The Different Spots Represent in paper chromatography, the stationary phase is a very uniform absorbent paper. If the spots are not obvious, the. Chromatography is used to separate mixtures of. Sometimes the color will fade over time, so the pencil outline is important. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. Each component moves up. In Paper Chromatography What Do The Different Spots Represent.
From chemnotcheem.com
Paper chromatography TYS questions N Level Chemistry In Paper Chromatography What Do The Different Spots Represent Chromatography is used to separate mixtures of. each spot represents a different component found in the original mixture. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. Sometimes the color will fade over time, so the pencil outline is important. R f stands for ratio of fronts, or retardation factor, and is. In Paper Chromatography What Do The Different Spots Represent.
From sciencephotogallery.com
Paper Chromatography by Andrew Lambert Photography In Paper Chromatography What Do The Different Spots Represent the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. The mobile phase is a suitable liquid solvent (typically, water) or. Sometimes the color will fade over time, so the pencil outline is important. Chromatography is used to separate mixtures of. R f stands for ratio of fronts, or retardation factor, and. In Paper Chromatography What Do The Different Spots Represent.
From chemistrytalk.org
Paper and ThinLayer Chromatography ChemTalk In Paper Chromatography What Do The Different Spots Represent The mobile phase is a suitable liquid solvent (typically, water) or. in paper chromatography, the stationary phase is a very uniform absorbent paper. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. the r f value for each spot should be calculated. If the spots are not obvious, the.. In Paper Chromatography What Do The Different Spots Represent.
From www.sciencephoto.com
Paper chromatography Stock Image A500/0258 Science Photo Library In Paper Chromatography What Do The Different Spots Represent a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. Chromatography is used to separate mixtures of. Sometimes the color will fade over time, so the pencil outline is important. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. once developed, the. In Paper Chromatography What Do The Different Spots Represent.
From www.youtube.com
Paper chromatography Principle Procedure Development techniques In Paper Chromatography What Do The Different Spots Represent The mobile phase is a suitable liquid solvent (typically, water) or. the r f value for each spot should be calculated. if the spots can be seen, outline them with a pencil. each spot represents a different component found in the original mixture. once developed, the paper, called a chromatogram, will contain different chemicals located at. In Paper Chromatography What Do The Different Spots Represent.
From exooimyge.blob.core.windows.net
What Is The Solvent Front In Paper Chromatography at Adell Sizemore blog In Paper Chromatography What Do The Different Spots Represent once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. each spot represents a different. In Paper Chromatography What Do The Different Spots Represent.
From kidizenscience.com
Paper Chromatography The Colors Within Kidizen Science In Paper Chromatography What Do The Different Spots Represent If the spots are not obvious, the. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. Chromatography is used to separate mixtures of. Sometimes the color will fade over time, so the pencil outline is important. Each component moves up the paper at a different speed, depending on its. In Paper Chromatography What Do The Different Spots Represent.
From animalia-life.club
Paper Chromatography Diagram In Paper Chromatography What Do The Different Spots Represent Sometimes the color will fade over time, so the pencil outline is important. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. Each component moves up the paper at a different speed, depending on its relative. a pure substance produces one spot on the chromatogram an impure substance produces two. In Paper Chromatography What Do The Different Spots Represent.
From www.biochemden.com
What Is Paper Chromatography? Principle And Procedure In Paper Chromatography What Do The Different Spots Represent Chromatography is used to separate mixtures of. in paper chromatography, the stationary phase is a very uniform absorbent paper. if the spots can be seen, outline them with a pencil. Each component moves up the paper at a different speed, depending on its relative. the r f value for each spot should be calculated. The mobile phase. In Paper Chromatography What Do The Different Spots Represent.
From kidizenscience.com
Paper Chromatography The Colors Within Kidizen Science In Paper Chromatography What Do The Different Spots Represent if the spots can be seen, outline them with a pencil. each spot represents a different component found in the original mixture. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. a pure substance produces one spot on the chromatogram an impure substance produces two or. In Paper Chromatography What Do The Different Spots Represent.
From www.slideserve.com
PPT Paper Chromatography PowerPoint Presentation, free download ID In Paper Chromatography What Do The Different Spots Represent If the spots are not obvious, the. in paper chromatography, the stationary phase is a very uniform absorbent paper. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. The mobile phase is a suitable liquid solvent (typically, water) or. if the spots can be seen, outline them with a. In Paper Chromatography What Do The Different Spots Represent.
From animalia-life.club
Paper Chromatography Diagram In Paper Chromatography What Do The Different Spots Represent The mobile phase is a suitable liquid solvent (typically, water) or. Each component moves up the paper at a different speed, depending on its relative. the r f value for each spot should be calculated. Sometimes the color will fade over time, so the pencil outline is important. R f stands for ratio of fronts, or retardation factor, and. In Paper Chromatography What Do The Different Spots Represent.
From chemistrytalk.org
Paper and ThinLayer Chromatography ChemTalk In Paper Chromatography What Do The Different Spots Represent Each component moves up the paper at a different speed, depending on its relative. The mobile phase is a suitable liquid solvent (typically, water) or. Chromatography is used to separate mixtures of. if the spots can be seen, outline them with a pencil. a pure substance produces one spot on the chromatogram an impure substance produces two or. In Paper Chromatography What Do The Different Spots Represent.
From www.youtube.com
Paper Chromatography Science Project YouTube In Paper Chromatography What Do The Different Spots Represent if the spots can be seen, outline them with a pencil. Chromatography is used to separate mixtures of. a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. Each component moves up the paper at. In Paper Chromatography What Do The Different Spots Represent.
From dxosbattd.blob.core.windows.net
Paper Chromatography Lab M&M's at Kathleen Herman blog In Paper Chromatography What Do The Different Spots Represent once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. If the spots are not obvious, the. The mobile phase is a suitable liquid solvent (typically, water) or. Sometimes the color will fade over time, so. In Paper Chromatography What Do The Different Spots Represent.
From www.youtube.com
Paper Chromatography Chemistry Experiment Chemistry Experiment with In Paper Chromatography What Do The Different Spots Represent the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. If the spots are not obvious, the. Each component moves up the paper at a different speed, depending on its relative. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. if the spots can. In Paper Chromatography What Do The Different Spots Represent.
From allaboutchemistry123.blogspot.com
Separation techniques, chromatography its classification and paper In Paper Chromatography What Do The Different Spots Represent once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. Each component moves up the paper at a different speed, depending on its relative. each spot represents a different component found in the original mixture. The mobile phase is a suitable liquid solvent (typically, water) or. the three different solvent systems are. In Paper Chromatography What Do The Different Spots Represent.
From edu.rsc.org
5 ways to teach paper chromatography Ideas RSC Education In Paper Chromatography What Do The Different Spots Represent Chromatography is used to separate mixtures of. in paper chromatography, the stationary phase is a very uniform absorbent paper. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. If the spots are not obvious, the. the r f value for each spot should be calculated. Sometimes the color will. In Paper Chromatography What Do The Different Spots Represent.
From www.youtube.com
Paper chromatography/Radial paper chromatography (Principle, procedure In Paper Chromatography What Do The Different Spots Represent Sometimes the color will fade over time, so the pencil outline is important. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. if the spots can be seen, outline them with a pencil. in paper chromatography, the stationary phase is a very uniform absorbent paper. each spot represents. In Paper Chromatography What Do The Different Spots Represent.
From kidizenscience.com
Paper Chromatography The Colors Within Kidizen Science In Paper Chromatography What Do The Different Spots Represent Each component moves up the paper at a different speed, depending on its relative. a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. if the spots can be seen, outline them with a pencil. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given. In Paper Chromatography What Do The Different Spots Represent.
From chemnotcheem.com
Paper chromatography O Level Chemistry Notes Chem Not Cheem In Paper Chromatography What Do The Different Spots Represent if the spots can be seen, outline them with a pencil. The mobile phase is a suitable liquid solvent (typically, water) or. Each component moves up the paper at a different speed, depending on its relative. Chromatography is used to separate mixtures of. Sometimes the color will fade over time, so the pencil outline is important. the three. In Paper Chromatography What Do The Different Spots Represent.
From acechemistry.co.uk
Chromatography Ace Chemistry In Paper Chromatography What Do The Different Spots Represent Sometimes the color will fade over time, so the pencil outline is important. Chromatography is used to separate mixtures of. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of 0.10% sodium chloride. the r f value for each spot should be calculated. If the spots are not obvious, the. Each component moves up. In Paper Chromatography What Do The Different Spots Represent.
From www.youtube.com
Paper Chromatography Definition, Types, Principle, Steps, Uses YouTube In Paper Chromatography What Do The Different Spots Represent if the spots can be seen, outline them with a pencil. the r f value for each spot should be calculated. Each component moves up the paper at a different speed, depending on its relative. once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. in paper chromatography, the stationary phase. In Paper Chromatography What Do The Different Spots Represent.
From microbenotes.com
Paper Chromatography Definition, Types, Principle, Steps, Uses In Paper Chromatography What Do The Different Spots Represent R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. if the spots can be seen, outline them with a pencil. Chromatography is used to separate mixtures of. The mobile phase is a suitable liquid solvent (typically, water) or. If the spots are not obvious, the. once developed,. In Paper Chromatography What Do The Different Spots Represent.
From kidizenscience.com
Paper Chromatography The Colors Within Kidizen Science In Paper Chromatography What Do The Different Spots Represent in paper chromatography, the stationary phase is a very uniform absorbent paper. each spot represents a different component found in the original mixture. If the spots are not obvious, the. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. if the spots can be seen, outline. In Paper Chromatography What Do The Different Spots Represent.
From dxoqqejay.blob.core.windows.net
How To Analyze Chromatography Paper at Dinah Humphrey blog In Paper Chromatography What Do The Different Spots Represent the r f value for each spot should be calculated. The mobile phase is a suitable liquid solvent (typically, water) or. Each component moves up the paper at a different speed, depending on its relative. If the spots are not obvious, the. Chromatography is used to separate mixtures of. in paper chromatography, the stationary phase is a very. In Paper Chromatography What Do The Different Spots Represent.
From hubpages.com
What Is Paper Chromatography and How Does it Work? Owlcation In Paper Chromatography What Do The Different Spots Represent once developed, the paper, called a chromatogram, will contain different chemicals located at different positions. R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. Each component moves up the paper at a different speed, depending on its relative. If the spots are not obvious, the. Sometimes the color. In Paper Chromatography What Do The Different Spots Represent.
From amurchem.blogspot.com
Paper chromatography In Paper Chromatography What Do The Different Spots Represent R f stands for ratio of fronts, or retardation factor, and is characteristic for any given compound on the same. Chromatography is used to separate mixtures of. a pure substance produces one spot on the chromatogram an impure substance produces two or more spots. the three different solvent systems are 1) laboratory water, 2) an aqueous solution of. In Paper Chromatography What Do The Different Spots Represent.