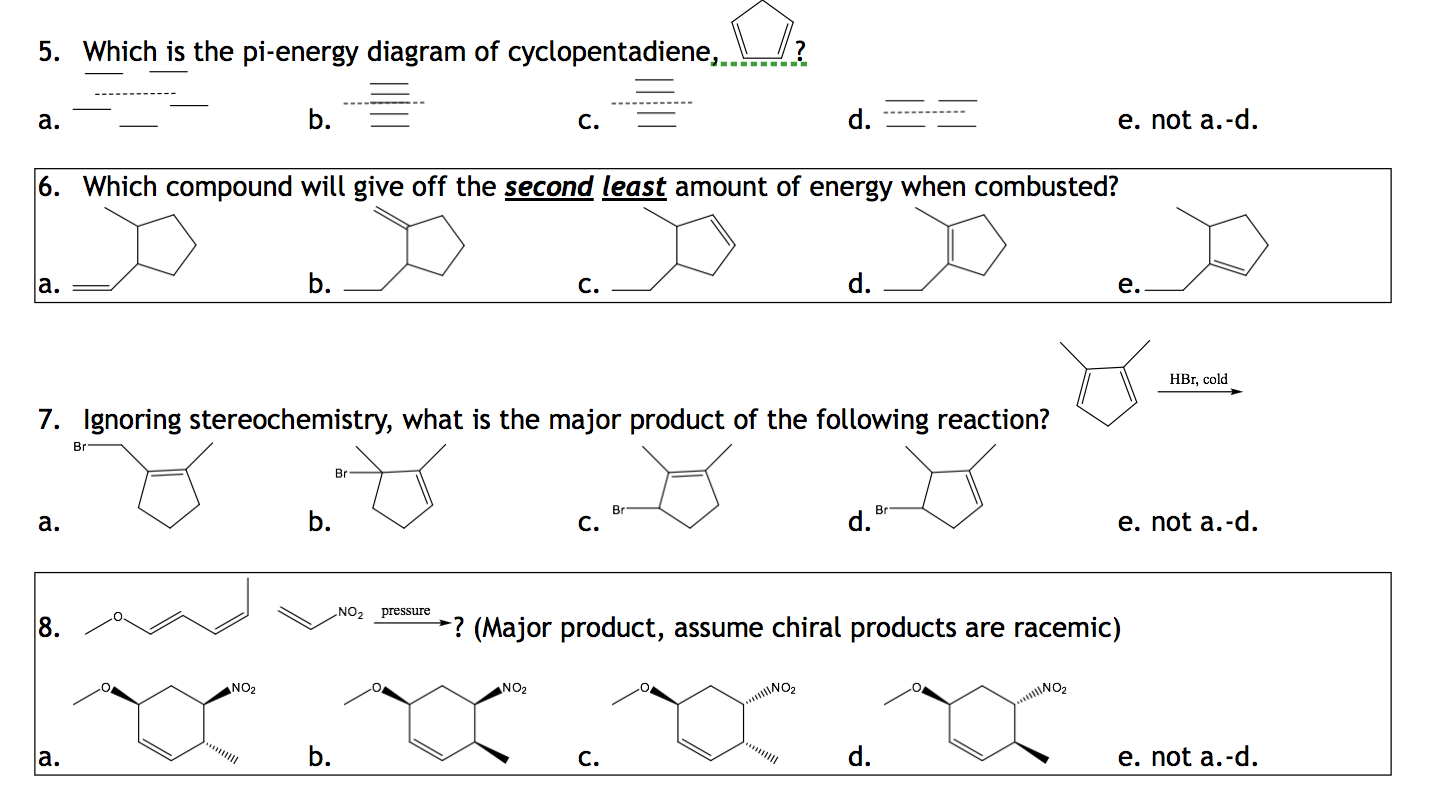
Pi Energy Definition . Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom.
from www.chegg.com
The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds.
Solved Which is the pienergy diagram of cyclopentadiene,
Pi Energy Definition Sigma and pi bonds are formed by the overlap of atomic orbitals. Sigma and pi bonds are formed by the overlap of atomic orbitals. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. The pi bonds are almost always weaker than sigma bonds.
From www.pienergy.com
Previous Processing Updates — PI Energy site Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of. Pi Energy Definition.
From www.pienergy.com
PI Energy on Electric Vehicles — PI Energy site Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. The pi bonds are almost always weaker than. Pi Energy Definition.
From www.pienergy.com
PI Energy’s Development Milestone Plan — PI Energy site Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.
From www.youtube.com
Raspberry Pi PICO Based Energy Meter Using IOT YouTube Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.
From www.adda247.com
Value of Pi in Fraction, Angle, and Decimal Pi Energy Definition Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from. Pi Energy Definition.
From raspberrypi.stackexchange.com
pi 2 Raspberry Pi 3 vs Pi 2 power consumption and heat dissipation Pi Energy Definition Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.
From www.dreamstime.com
Pi definition stock image. Image of circle, blackboard 27160373 Pi Energy Definition Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.
From 9to5science.com
[Solved] Draw a simplified MO diagram for the pi system 9to5Science Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The pi bonds are almost always weaker than. Pi Energy Definition.
From www.expii.com
Sigma and Pi Bonds — Definition & Overview Expii Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from. Pi Energy Definition.
From www.slideserve.com
PPT Facts on Pi PowerPoint Presentation, free download ID2484781 Pi Energy Definition Sigma and pi bonds are formed by the overlap of atomic orbitals. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other. Pi Energy Definition.
From www.researchgate.net
3 Typical energy level of pielectron organic molecules. Download Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.
From www.thephysicspoint.com
What is Energy? Definition, Formula, Unit, Types and Example Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from. Pi Energy Definition.
From www.pienergy.com
IPCC Climate Report and PI Energy — PI Energy site Pi Energy Definition Sigma and pi bonds are formed by the overlap of atomic orbitals. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other. Pi Energy Definition.
From www.pienergy.com
The Future of Solar Manufacturing with PI Energy — PI Energy site Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from. Pi Energy Definition.
From www.etsy.com
Pi the Definition Classroom Math Poster Etsy Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of. Pi Energy Definition.
From www.youtube.com
What is pi? pi value of pi Define pi. YouTube Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.
From www.pienergy.com
PI Energy News — PI Energy site Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the. Pi Energy Definition.
From www.dreamstime.com
Pi definition stock illustration. Illustration of circle 50478117 Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of. Pi Energy Definition.
From www.youtube.com
Electron Energetics of Pi Systems and Absorption of Light YouTube Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The pi bonds are almost always weaker than. Pi Energy Definition.
From www.sciencefacts.net
Chemical Energy Definition, Facts, Examples, and Pictures Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the. Pi Energy Definition.
From www.researchgate.net
Slope and offset terms of the relation between PHA and PI energy Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.
From www.livescience.com
What Is Pi? Live Science Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other. Pi Energy Definition.
From getuhoo.com
uHoo and Pi Energy a Partnership to Bring Health, Energy Savings Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of. Pi Energy Definition.
From pisquare.osisoft.com
ISO50001 Energy Management with the PI System Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other. Pi Energy Definition.
From www.researchgate.net
3 Typical energy level of pielectron organic molecules. Download Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. Two pi bonds are formed from. Pi Energy Definition.
From byjus.com
Value of Pi in Maths Definition, Forms & Solved Examples Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. The pi bonds are almost always weaker than. Pi Energy Definition.
From www.researchgate.net
Plot of observed values (1/LD25) vs. the descriptor Pi Energy. The Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other. Pi Energy Definition.
From www.pienergy.com
PI Energy Advantages — PI Energy site Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from. Pi Energy Definition.
From en.wikipedia.org
Pi Wikipedia Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The pi bonds are almost always weaker than. Pi Energy Definition.
From quizlet.com
Thermal, and Potential Energy Diagram Quizlet Pi Energy Definition Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from. Pi Energy Definition.
From www.fphc.com
Lopezled Pi Energy, Climargy energy efficiency partnership Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Sigma and pi bonds are formed by the overlap of atomic orbitals. The pi bonds are almost always weaker than sigma bonds. Two pi bonds are formed from. Pi Energy Definition.
From www.yellow-pages.ph
Photos of Pi Energy Inc. in Pasig City, Metro Manila Yellow Pages PH Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of. Pi Energy Definition.
From www.citybiz.co
PI Energy Announces Pilot Agreement with Acento Real Estate Partners Pi Energy Definition The pi bonds are almost always weaker than sigma bonds. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Sigma and pi bonds are formed by the overlap of atomic orbitals. Two pi bonds are formed from. Pi Energy Definition.
From www.dreamstime.com
Pi definition poster stock illustration. Illustration of chalk 139385108 Pi Energy Definition The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and pi 8 *) have unique energy levels, while the other six form degenerate pairs. Sigma and pi bonds are formed by the overlap of atomic orbitals. Two pi bonds are formed from the lateral overlap of the 2p y and 2p z. Pi Energy Definition.
From www.chegg.com
Solved Which is the pienergy diagram of cyclopentadiene, Pi Energy Definition Two pi bonds are formed from the lateral overlap of the 2p y and 2p z orbitals of carbon atom. The pi bonds are almost always weaker than sigma bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals. The result of molecular orbital calculations tells us that the lowest and highest energy mos (pi 1 and. Pi Energy Definition.