Buffer Experiment Chemistry . There are two combinations of solutions that may. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. A solution of acetic acid. To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{h^+}\) into. Buffers are characterized by the ph range over. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). It is able to neutralize small. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. This characteristic makes buffers important in biological and.
from www.slideshare.net
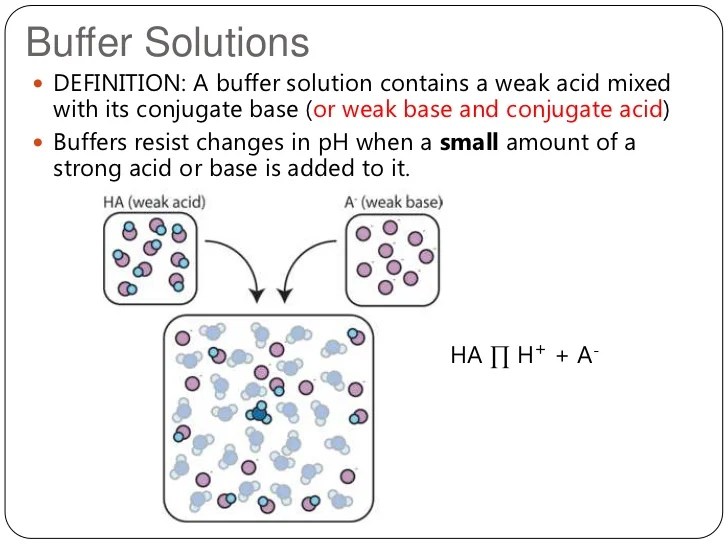
There are two combinations of solutions that may. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. This characteristic makes buffers important in biological and. A solution of acetic acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution.
2012 topic 18 2 buffer solutions
Buffer Experiment Chemistry There are two combinations of solutions that may. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). Calculate the amounts of acid and base needed to prepare a buffer of a given ph. It is able to neutralize small. This characteristic makes buffers important in biological and. A solution of acetic acid. There are two combinations of solutions that may. Buffers are characterized by the ph range over. A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{h^+}\) into. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Experiment Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level. Buffer Experiment Chemistry.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Buffer Experiment Chemistry Buffers are characterized by the ph range over. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts. Buffer Experiment Chemistry.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation Buffer Experiment Chemistry There are two combinations of solutions that may. Buffers are characterized by the ph range over. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. Buffer Experiment Chemistry.
From www.tffn.net
How Does a Buffer Work? An InDepth Guide to Buffers and Their Buffer Experiment Chemistry Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. This characteristic makes buffers important in biological and. A buffer is a solution that resists changes. Buffer Experiment Chemistry.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Buffer Experiment Chemistry A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{h^+}\) into. Buffer solutions resist a change in ph when. Buffer Experiment Chemistry.
From animalia-life.club
Phosphate Buffer System Equation Buffer Experiment Chemistry Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. A buffer is a solution that maintains the stability of a system’s ph level when adding small. Buffer Experiment Chemistry.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Buffer Experiment Chemistry A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. Buffer solutions resist a change in ph when small amounts. Buffer Experiment Chemistry.
From www.flinnsci.ca
Buffers in Household Products Advanced Inquiry Laboratory Kit Buffer Experiment Chemistry This characteristic makes buffers important in biological and. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. There are two combinations of solutions that may. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. Buffer Experiment Chemistry.
From zanenewswaller.blogspot.com
Why Buffer Solution Is Used in Edta Titration Buffer Experiment Chemistry A solution of acetic acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and. Calculate the amounts of acid and. Buffer Experiment Chemistry.
From www.dreamstime.com
Making Buffer Solution Royalty Free Stock Photo Image 30119865 Buffer Experiment Chemistry There are two combinations of solutions that may. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are characterized by the ph range over. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A solution whose ph is not altered to any. Buffer Experiment Chemistry.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry Buffer Experiment Chemistry A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. A solution of acetic acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and. A buffer is a solution that maintains. Buffer Experiment Chemistry.
From dramarnathgiri.blogspot.com
EHSQ (Environment,Health,Safety and Quality) Chemistry of buffers and Buffer Experiment Chemistry Buffers are characterized by the ph range over. A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffer is a solution that can resist ph change upon the addition of an. Buffer Experiment Chemistry.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid Buffer Experiment Chemistry Buffers are characterized by the ph range over. A solution of acetic acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called. Buffer Experiment Chemistry.
From pdfprof.com
buffer capacity experiment procedure Buffer Experiment Chemistry It is able to neutralize small. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. A buffer is a solution. Buffer Experiment Chemistry.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer Experiment Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level. Buffer Experiment Chemistry.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Experiment Chemistry Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. There are two combinations of solutions that may. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution of acetic acid. To be a. Buffer Experiment Chemistry.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Buffer Experiment Chemistry Buffers are characterized by the ph range over. To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{h^+}\) into. This characteristic makes buffers important in biological and. Buffer solutions resist a change in ph when small amounts of. Buffer Experiment Chemistry.
From app.jove.com
Buffers, Buffer Components and Buffer Action Chemistry JoVe Buffer Experiment Chemistry Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). There are two combinations of solutions that may. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. A solution of acetic acid. Buffers are characterized. Buffer Experiment Chemistry.
From www.vernier.com
Buffers > Experiment 19 from Advanced Chemistry with Vernier Buffer Experiment Chemistry It is able to neutralize small. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. A solution of acetic acid. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution.. Buffer Experiment Chemistry.
From bid.meetbirmingham.com
O Sistema Detectou A Saturação De Um Buffer EDULEARN Buffer Experiment Chemistry A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. This characteristic makes buffers important in biological and. Buffer solutions resist a change in. Buffer Experiment Chemistry.
From 2012books.lardbucket.org
Buffers Buffer Experiment Chemistry Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. This characteristic makes buffers important in biological and. Buffers are characterized by the ph range over. A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. A. Buffer Experiment Chemistry.
From www.youtube.com
Preparation and Properties of Buffer Solution Chemical Equilibrium Buffer Experiment Chemistry A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. Buffer Experiment Chemistry.
From www.researchgate.net
(PDF) A Discovery Chemistry Experiment on Buffers Buffer Experiment Chemistry To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{h^+}\) into. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. There are two combinations of solutions that may. A buffer is. Buffer Experiment Chemistry.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Experiment Chemistry Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). There are two combinations of solutions that may. A solution of acetic acid. To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that. Buffer Experiment Chemistry.
From www.feedback-shop.co.uk
Buffer solutions Digital Protolysis equilibrium Acids and bases Buffer Experiment Chemistry A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. There are two combinations of solutions that may. This characteristic makes buffers important in biological and. A solution of acetic acid. A solution whose ph is not altered to any great extent by the addition of small quantities. Buffer Experiment Chemistry.
From sciencing.com
How to Make a Citric Acid Buffer Solution Sciencing Buffer Experiment Chemistry A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. Buffers are characterized by the ph range over. There are two combinations of solutions that may. This characteristic makes buffers important in biological and. Buffer solutions resist a change in ph when small amounts of a strong acid or. Buffer Experiment Chemistry.
From www.youtube.com
Chapter 16 Buffers Some Buffer Experiments with Household Items Buffer Experiment Chemistry A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution of acetic acid. Buffers are characterized by the ph range over. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. To be a. Buffer Experiment Chemistry.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Experiment Chemistry To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{h^+}\) into. It is able to neutralize small. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to. Buffer Experiment Chemistry.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Buffer Experiment Chemistry A buffer is a solution that resists changes to ph when a strong acid or base is added to the solution. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffers are characterized by the ph range over. To be a good buffer, a solution should have a component that acts as a. Buffer Experiment Chemistry.
From www.youtube.com
Lab 8 Acids, Bases, and Buffers experiment YouTube Buffer Experiment Chemistry Buffers are characterized by the ph range over. There are two combinations of solutions that may. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. This characteristic makes buffers important in biological and. Calculate the amounts of acid and base needed to. Buffer Experiment Chemistry.
From courses.lumenlearning.com
10.5 Buffers The Basics of General, Organic, and Biological Chemistry Buffer Experiment Chemistry A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and. There are two combinations of solutions that may. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able. Buffer Experiment Chemistry.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog Buffer Experiment Chemistry Calculate the amounts of acid and base needed to prepare a buffer of a given ph. There are two combinations of solutions that may. A solution of acetic acid. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer solutions resist. Buffer Experiment Chemistry.
From www.youtube.com
TRU Chemistry Labs Experiment Titration Curves Part B Buffer Systems Buffer Experiment Chemistry A solution of acetic acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. There are two combinations of solutions that may. Buffers lessen or absorb the drastic changes in ph that occur when small amounts of acids and bases are added to solution. To be a. Buffer Experiment Chemistry.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Experiment Chemistry This characteristic makes buffers important in biological and. To be a good buffer, a solution should have a component that acts as a base (takes \(\ce{h^+}\) out of solution) and a component that acts as an acid (puts more \(\ce{h^+}\) into. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph. Buffer Experiment Chemistry.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Experiment Chemistry It is able to neutralize small. Calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \(\pageindex{1}\)). A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffer Experiment Chemistry.