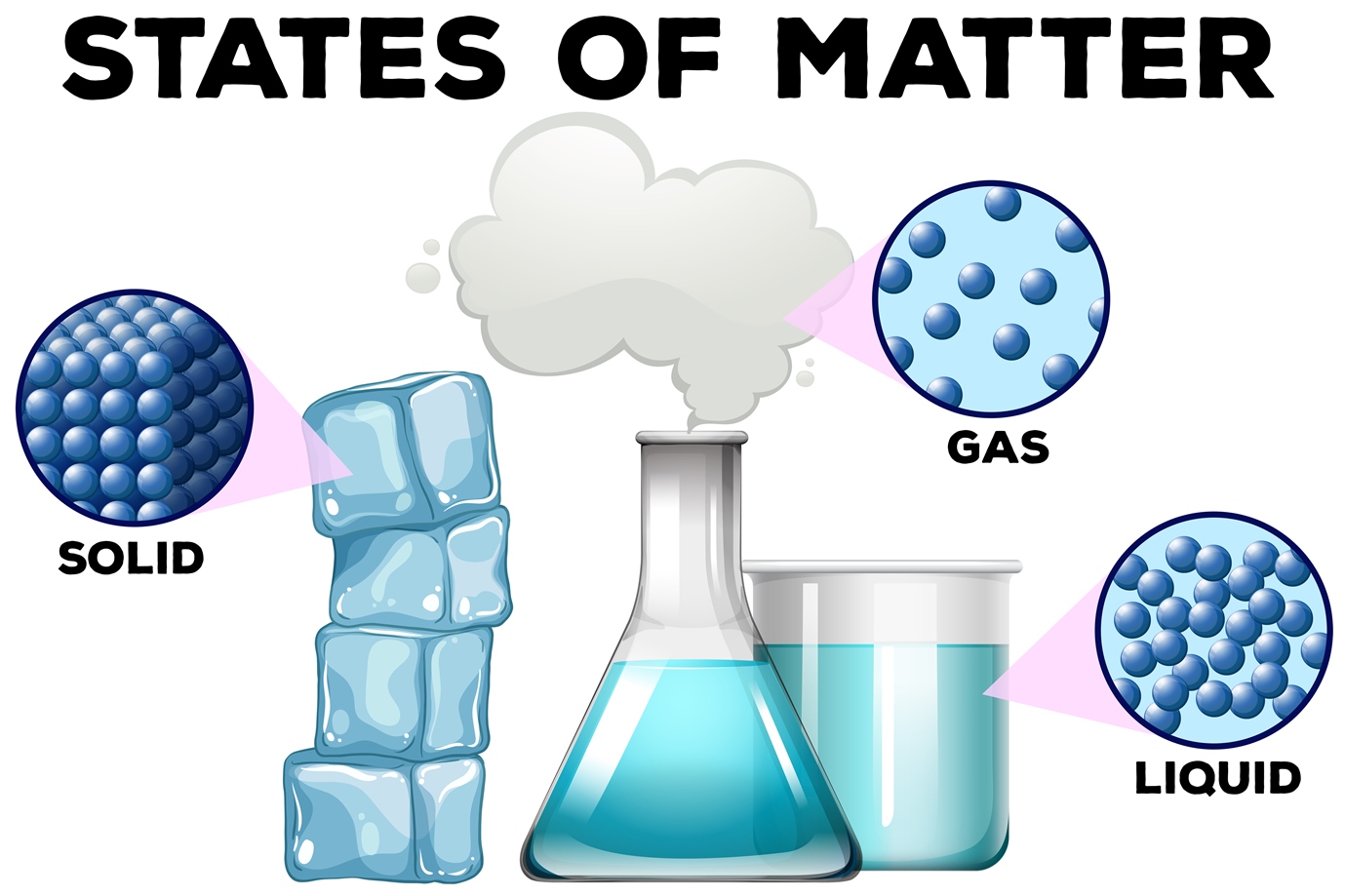
Solid Liquid Gas Highest Kinetic Energy . in general covalent bonds determine: Substances can exist in three states of. liquids have more kinetic energy than solids. If you add heat energy to a liquid, the particles will move faster around each. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. the gaseous state has the highest compressibility as compared to solids and liquids. The rate is diffusion is higher than solids and liquids. matter exists in three states: in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. a description of the appearance of a substance or how it acts without involving chemical reactions.
from exoptqlgc.blob.core.windows.net
We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. matter exists in three states: the gaseous state has the highest compressibility as compared to solids and liquids. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. Substances can exist in three states of. If you add heat energy to a liquid, the particles will move faster around each. liquids have more kinetic energy than solids. The rate is diffusion is higher than solids and liquids. in general covalent bonds determine:
Solids Liquids And Gases Atoms at Todd Spence blog
Solid Liquid Gas Highest Kinetic Energy a description of the appearance of a substance or how it acts without involving chemical reactions. matter exists in three states: for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. the gaseous state has the highest compressibility as compared to solids and liquids. a description of the appearance of a substance or how it acts without involving chemical reactions. Substances can exist in three states of. in general covalent bonds determine: in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. The rate is diffusion is higher than solids and liquids. liquids have more kinetic energy than solids. If you add heat energy to a liquid, the particles will move faster around each. We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid Liquid Gas Highest Kinetic Energy a description of the appearance of a substance or how it acts without involving chemical reactions. The rate is diffusion is higher than solids and liquids. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. liquids have more kinetic energy than solids. matter exists. Solid Liquid Gas Highest Kinetic Energy.
From exoptqlgc.blob.core.windows.net
Solids Liquids And Gases Atoms at Todd Spence blog Solid Liquid Gas Highest Kinetic Energy in general covalent bonds determine: matter exists in three states: The rate is diffusion is higher than solids and liquids. If you add heat energy to a liquid, the particles will move faster around each. Substances can exist in three states of. in terms of relative energy, gas particles have the most energy, solid particles have the. Solid Liquid Gas Highest Kinetic Energy.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes Solid Liquid Gas Highest Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each. in general covalent bonds determine: for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. a description of the appearance of a substance or how it acts without. Solid Liquid Gas Highest Kinetic Energy.
From byjus.com
Which of the following shows Highest interpartical spaces Highest Solid Liquid Gas Highest Kinetic Energy matter exists in three states: We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. Substances can exist in three states of. a description of the appearance. Solid Liquid Gas Highest Kinetic Energy.
From www.slideserve.com
PPT Density PowerPoint Presentation, free download ID5878733 Solid Liquid Gas Highest Kinetic Energy for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. the gaseous state has the highest compressibility as compared to solids and liquids. liquids have more kinetic energy than solids. The rate is diffusion is higher than solids and liquids. in general. Solid Liquid Gas Highest Kinetic Energy.
From www.tes.com
theory of solids, liquids and gases Teaching Resources Solid Liquid Gas Highest Kinetic Energy a description of the appearance of a substance or how it acts without involving chemical reactions. We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. Substances can exist in three states of. If you add heat energy to a liquid, the particles will move faster around each. The rate. Solid Liquid Gas Highest Kinetic Energy.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID2707404 Solid Liquid Gas Highest Kinetic Energy for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. If you add heat energy to a liquid, the particles will move faster around each. We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. . Solid Liquid Gas Highest Kinetic Energy.
From msreinders.weebly.com
States of Matter Shaw Middle School Science Solid Liquid Gas Highest Kinetic Energy for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. the gaseous state has the highest compressibility as compared to solids and. Solid Liquid Gas Highest Kinetic Energy.
From www.slideshare.net
Particle Theory (Slg Introduction) Solid Liquid Gas Highest Kinetic Energy for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. in terms of relative energy, gas particles have the most energy, solid particles have the. Solid Liquid Gas Highest Kinetic Energy.
From www.sliderbase.com
Molecular Theory Presentation Chemistry Solid Liquid Gas Highest Kinetic Energy the gaseous state has the highest compressibility as compared to solids and liquids. liquids have more kinetic energy than solids. The rate is diffusion is higher than solids and liquids. matter exists in three states: in general covalent bonds determine: for example, converting a liquid, in which the molecules are close together, to a gas,. Solid Liquid Gas Highest Kinetic Energy.
From slideplayer.com
Solid Gas Liquid INTERNAL ENERGY, U ppt download Solid Liquid Gas Highest Kinetic Energy the gaseous state has the highest compressibility as compared to solids and liquids. Substances can exist in three states of. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. a description of the appearance of a substance or how it acts without. Solid Liquid Gas Highest Kinetic Energy.
From x-engineer.org
Which are the states of matter Solid Liquid Gas Highest Kinetic Energy the gaseous state has the highest compressibility as compared to solids and liquids. If you add heat energy to a liquid, the particles will move faster around each. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. a description of the appearance. Solid Liquid Gas Highest Kinetic Energy.
From brainly.in
How does the energy of solids, liquids, and gases compare Solid Liquid Gas Highest Kinetic Energy matter exists in three states: a description of the appearance of a substance or how it acts without involving chemical reactions. If you add heat energy to a liquid, the particles will move faster around each. the gaseous state has the highest compressibility as compared to solids and liquids. We will also examine how the kinetic theory. Solid Liquid Gas Highest Kinetic Energy.
From slideplayer.com
Target 13 Thursday, September 21, ppt download Solid Liquid Gas Highest Kinetic Energy We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. the gaseous state has the highest compressibility as compared to solids and liquids. in general covalent bonds. Solid Liquid Gas Highest Kinetic Energy.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Gas Highest Kinetic Energy the gaseous state has the highest compressibility as compared to solids and liquids. matter exists in three states: liquids have more kinetic energy than solids. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. The rate is diffusion is higher than solids and liquids.. Solid Liquid Gas Highest Kinetic Energy.
From www.youtube.com
theory of Solids, Liquids & Gases IGCSE Chemistry Learn Solid Liquid Gas Highest Kinetic Energy We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. a description of the appearance of a substance or how it acts without involving chemical reactions. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. the. Solid Liquid Gas Highest Kinetic Energy.
From gcsephysicsninja.com
3. Energy of solids, liquids and gases Solid Liquid Gas Highest Kinetic Energy Substances can exist in three states of. liquids have more kinetic energy than solids. a description of the appearance of a substance or how it acts without involving chemical reactions. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. the gaseous. Solid Liquid Gas Highest Kinetic Energy.
From dxohczxqw.blob.core.windows.net
Particles In Solids Liquids And Gases The Same at Charles Guenther blog Solid Liquid Gas Highest Kinetic Energy a description of the appearance of a substance or how it acts without involving chemical reactions. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. in terms of relative energy, gas particles have the most energy, solid particles have the least energy. Solid Liquid Gas Highest Kinetic Energy.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID5445725 Solid Liquid Gas Highest Kinetic Energy in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. The rate is diffusion is higher than solids and liquids. Substances can exist in three states of. liquids have more kinetic energy than solids. a description of the appearance of a substance or how it acts. Solid Liquid Gas Highest Kinetic Energy.
From thehungryjpeg.com
Different states of matter solid, liquid, gas vector diagram By Solid Liquid Gas Highest Kinetic Energy the gaseous state has the highest compressibility as compared to solids and liquids. The rate is diffusion is higher than solids and liquids. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. liquids have more kinetic energy than solids. Substances can exist in three states. Solid Liquid Gas Highest Kinetic Energy.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid Liquid Gas Highest Kinetic Energy matter exists in three states: liquids have more kinetic energy than solids. in general covalent bonds determine: the gaseous state has the highest compressibility as compared to solids and liquids. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. . Solid Liquid Gas Highest Kinetic Energy.
From www.slideserve.com
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint Solid Liquid Gas Highest Kinetic Energy We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. the gaseous state has the highest compressibility as compared to solids and liquids. Substances can exist in three states of. liquids have more kinetic energy than solids. in general covalent bonds determine: matter exists in three states:. Solid Liquid Gas Highest Kinetic Energy.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation Solid Liquid Gas Highest Kinetic Energy liquids have more kinetic energy than solids. in general covalent bonds determine: in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. a description of the appearance of a substance or how it acts without involving chemical reactions. the gaseous state has the highest. Solid Liquid Gas Highest Kinetic Energy.
From www.elevise.co.uk
P3 E) States of Matter AQA Combined Science Trilogy Elevise Solid Liquid Gas Highest Kinetic Energy We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. for example, converting a liquid, in which the molecules are close together, to a gas, in which the. Solid Liquid Gas Highest Kinetic Energy.
From gcse-revision-chemistry.blogspot.com
Beccy's Chemistry Revision 2018 Section 1 a) Summary Solid Liquid Gas Highest Kinetic Energy in general covalent bonds determine: matter exists in three states: We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. Substances can exist in three states of. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are.. Solid Liquid Gas Highest Kinetic Energy.
From www.slideserve.com
PPT Solids, Liquids, and Gases PowerPoint Presentation, free download Solid Liquid Gas Highest Kinetic Energy We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. If you add heat energy to a liquid, the particles will move faster around each. liquids have more kinetic energy than solids. Substances can exist in three states of. a description of the appearance of a substance or how. Solid Liquid Gas Highest Kinetic Energy.
From www.freeexamacademy.com
The Particulate Nature Of Matter IGCSE Chemistry Free Exam Academy Solid Liquid Gas Highest Kinetic Energy in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. liquids have more kinetic energy than solids. The rate is diffusion is higher than solids and liquids. the gaseous state has the highest compressibility as compared to solids and liquids. in general covalent bonds determine:. Solid Liquid Gas Highest Kinetic Energy.
From diagramlibkutshase6.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid Liquid Gas Highest Kinetic Energy If you add heat energy to a liquid, the particles will move faster around each. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. matter exists in three states: The rate is diffusion is higher than solids and liquids. We will also examine. Solid Liquid Gas Highest Kinetic Energy.
From exocprdpx.blob.core.windows.net
Solid Liquid And Gas Particles at Mui Jefferson blog Solid Liquid Gas Highest Kinetic Energy in general covalent bonds determine: The rate is diffusion is higher than solids and liquids. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. Substances can exist in three states of. matter exists in three states: We will also examine how the. Solid Liquid Gas Highest Kinetic Energy.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid Liquid Gas Highest Kinetic Energy for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. a description of the appearance of a substance or how it acts without involving chemical reactions. If you add heat energy to a liquid, the particles will move faster around each. The rate is. Solid Liquid Gas Highest Kinetic Energy.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions Solid Liquid Gas Highest Kinetic Energy liquids have more kinetic energy than solids. in terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are. matter exists in three states: the gaseous state has the highest compressibility as compared to solids and liquids. We will also examine how the kinetic theory of matter. Solid Liquid Gas Highest Kinetic Energy.
From www.pinterest.com
States of matter can be more than just your average solids, liquids and Solid Liquid Gas Highest Kinetic Energy liquids have more kinetic energy than solids. matter exists in three states: Substances can exist in three states of. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. The rate is diffusion is higher than solids and liquids. in terms of. Solid Liquid Gas Highest Kinetic Energy.
From www.docbrown.info
GASES LIQUIDS SOLIDS States of Matter, particle theory models Solid Liquid Gas Highest Kinetic Energy a description of the appearance of a substance or how it acts without involving chemical reactions. matter exists in three states: the gaseous state has the highest compressibility as compared to solids and liquids. We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. The rate is diffusion. Solid Liquid Gas Highest Kinetic Energy.
From www.turito.com
States of Matter Solids, Liquids, Gases, and Plasma Solid Liquid Gas Highest Kinetic Energy for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart,. a description of the appearance of a substance or how it acts without involving chemical reactions. If you add heat energy to a liquid, the particles will move faster around each. the gaseous. Solid Liquid Gas Highest Kinetic Energy.
From www.shutterstock.com
Theory Matter Infographic Diagram Showing Stock Illustration Solid Liquid Gas Highest Kinetic Energy in general covalent bonds determine: We will also examine how the kinetic theory of matter helps explain boiling and melting points as well as. If you add heat energy to a liquid, the particles will move faster around each. for example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules. Solid Liquid Gas Highest Kinetic Energy.