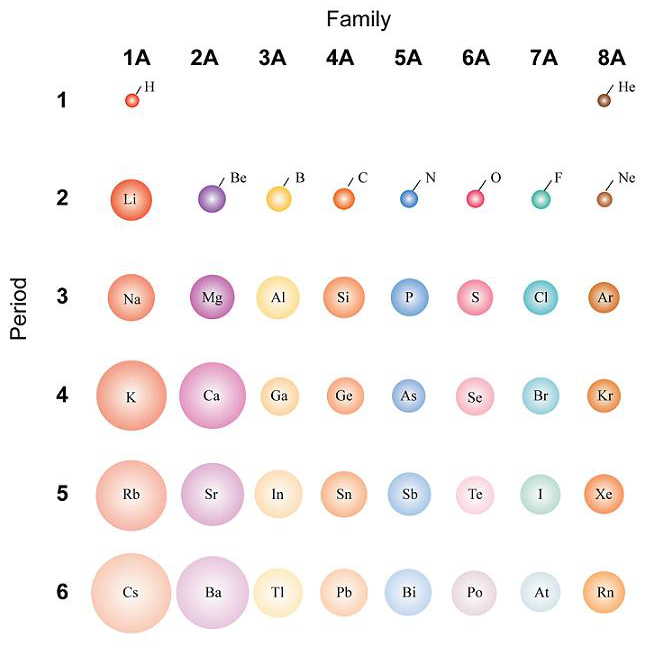
How To Calculate The Diameter Of An Atom . The covalent radius of a chlorine atom,. a variety of methods have been established to measure the size of a single atom or ion. This table shows how the atom size, and atomic radius. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. the plural of nucleus is nuclei. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. atom size values are calculated from atomic radius data. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. The covalent atomic radius ( r cov ) is half. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. Atoms which have the same.
from www.coursehero.com
atom size values are calculated from atomic radius data. The covalent atomic radius ( r cov ) is half. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. a variety of methods have been established to measure the size of a single atom or ion. the plural of nucleus is nuclei. This table shows how the atom size, and atomic radius. The covalent radius of a chlorine atom,. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.
Atomic Size Introduction to Chemistry Course Hero
How To Calculate The Diameter Of An Atom The covalent radius of a chlorine atom,. The covalent radius of a chlorine atom,. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The covalent atomic radius ( r cov ) is half. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. the plural of nucleus is nuclei. This table shows how the atom size, and atomic radius. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. atom size values are calculated from atomic radius data. Atoms which have the same. a variety of methods have been established to measure the size of a single atom or ion.
From www.nagwa.com
Question Video Identifying the Expression That Describes the Size of How To Calculate The Diameter Of An Atom Atoms which have the same. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. This table shows how the atom size, and atomic radius. a variety of methods have been established to measure the size of a single atom or ion. Of an atom is less than \. How To Calculate The Diameter Of An Atom.
From www.cuemath.com
Diameter of a Circle Formula, Examples Diameter Formula How To Calculate The Diameter Of An Atom the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. a variety of methods have been established to measure the size of a single atom or ion. Atoms which. How To Calculate The Diameter Of An Atom.
From www.chem.fsu.edu
Electron Configurations How To Calculate The Diameter Of An Atom the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The covalent radius of a chlorine atom,. a variety of methods have been established to measure the size of a single atom or ion. atom size values are calculated from atomic radius data. the plural of nucleus. How To Calculate The Diameter Of An Atom.
From www.youtube.com
How To Calculate The Number of Atoms Chemistry YouTube How To Calculate The Diameter Of An Atom the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. Atoms which have the same. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. the plural of nucleus is nuclei. The covalent atomic. How To Calculate The Diameter Of An Atom.
From www.youtube.com
The relation between lattice constant a and atomic radius r for Diamond How To Calculate The Diameter Of An Atom the plural of nucleus is nuclei. a variety of methods have been established to measure the size of a single atom or ion. atom size values are calculated from atomic radius data. The covalent atomic radius ( r cov ) is half. Atoms which have the same. The covalent radius of a chlorine atom,. the size. How To Calculate The Diameter Of An Atom.
From www.coursehero.com
Atomic Size Introduction to Chemistry Course Hero How To Calculate The Diameter Of An Atom This table shows how the atom size, and atomic radius. Atoms which have the same. The covalent radius of a chlorine atom,. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. the plural. How To Calculate The Diameter Of An Atom.
From www.numerade.com
SOLVED A typical atom has a diameter of about 1.0x10−10m (a) What is How To Calculate The Diameter Of An Atom a variety of methods have been established to measure the size of a single atom or ion. Atoms which have the same. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. the plural of nucleus is nuclei. The covalent atomic radius ( r cov ) is half. atom size values are. How To Calculate The Diameter Of An Atom.
From www.chegg.com
Solved An atom has a diameter of 5.00 Å and the nucleus of How To Calculate The Diameter Of An Atom the plural of nucleus is nuclei. a variety of methods have been established to measure the size of a single atom or ion. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The covalent atomic radius ( r cov ) is half. the size of atoms. How To Calculate The Diameter Of An Atom.
From www.youtube.com
How to calculate the RADIUS, DIAMETER and the CIRCUMFERENCE of a circle How To Calculate The Diameter Of An Atom The covalent radius of a chlorine atom,. a variety of methods have been established to measure the size of a single atom or ion. atom size values are calculated from atomic radius data. Atoms which have the same. The covalent atomic radius ( r cov ) is half. Of an atom is less than \ (\frac {1} {10,000}\). How To Calculate The Diameter Of An Atom.
From www.numerade.com
An electron is confined to a linear region with a length of the same How To Calculate The Diameter Of An Atom The covalent radius of a chlorine atom,. The covalent atomic radius ( r cov ) is half. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. This table shows how the atom size, and atomic radius. the size of an atom can be. How To Calculate The Diameter Of An Atom.
From www.numerade.com
SOLVEDThe diameter of a hydrogen atom is about 0.1 nm, and the How To Calculate The Diameter Of An Atom the plural of nucleus is nuclei. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. Atoms which have the same. The covalent atomic radius ( r cov ) is half. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. the size. How To Calculate The Diameter Of An Atom.
From www.coursehero.com
[Solved] Using the diameter of an atom, How many atoms stack on top of How To Calculate The Diameter Of An Atom This table shows how the atom size, and atomic radius. The covalent radius of a chlorine atom,. The covalent atomic radius ( r cov ) is half. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. a variety of methods have been established to measure the size of a single atom or ion.. How To Calculate The Diameter Of An Atom.
From www.slideshare.net
Basic Atomic Structure How To Calculate The Diameter Of An Atom The covalent radius of a chlorine atom,. The covalent atomic radius ( r cov ) is half. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. This table shows how the atom size, and atomic radius. the plural of nucleus is nuclei. Of an atom is less than. How To Calculate The Diameter Of An Atom.
From www.youtube.com
The diameter of zinc atom is 2.6 Å. Calculate (a) radius of zinc atom How To Calculate The Diameter Of An Atom atom size values are calculated from atomic radius data. Atoms which have the same. the plural of nucleus is nuclei. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. a variety of methods have been established to measure the size of a single atom or ion.. How To Calculate The Diameter Of An Atom.
From www.youtube.com
Finding radius and volume of atom from density and molar mass YouTube How To Calculate The Diameter Of An Atom Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The covalent radius of a chlorine atom,. atom size values are calculated from atomic radius data. Atoms which have the same. This table shows how the atom size, and atomic radius. atomic radii can be obtained from quantum mechanical calculations or can be. How To Calculate The Diameter Of An Atom.
From 9to5science.com
[Solved] How do I find the diameter of an aluminum atom 9to5Science How To Calculate The Diameter Of An Atom The covalent atomic radius ( r cov ) is half. This table shows how the atom size, and atomic radius. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. atom size values are calculated from atomic radius data. The covalent radius of a chlorine atom,. the size of an atom can be. How To Calculate The Diameter Of An Atom.
From www.branchor.com
How to Calculate Diameter A Comprehensive Guide for Beginners The How To Calculate The Diameter Of An Atom atom size values are calculated from atomic radius data. The covalent radius of a chlorine atom,. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of. How To Calculate The Diameter Of An Atom.
From www.numerade.com
SOLVEDThe diameter of an atom is 1.2 \times 10^{10} \mathrm{m} and How To Calculate The Diameter Of An Atom Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. The covalent atomic radius ( r cov ) is half. This table shows how the atom size, and atomic radius. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid.. How To Calculate The Diameter Of An Atom.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID60853 How To Calculate The Diameter Of An Atom a variety of methods have been established to measure the size of a single atom or ion. This table shows how the atom size, and atomic radius. the plural of nucleus is nuclei. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. atom size values are calculated from atomic radius data.. How To Calculate The Diameter Of An Atom.
From www.wikihow.com
How to Find the Diameter of a Circle wikiHow How To Calculate The Diameter Of An Atom the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. This table shows how the atom size, and atomic radius. Atoms which have the same. the size of atoms can be estimated with the. How To Calculate The Diameter Of An Atom.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation How To Calculate The Diameter Of An Atom atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. a variety of methods. How To Calculate The Diameter Of An Atom.
From www.slideserve.com
PPT Chapter Three PowerPoint Presentation, free download ID4330951 How To Calculate The Diameter Of An Atom the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. The covalent radius of a chlorine atom,. The covalent atomic radius ( r cov ) is half. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. a variety. How To Calculate The Diameter Of An Atom.
From www.coursehero.com
[Solved] The diameter of a hydrogen atom is about 1.05x10 10 meters. A How To Calculate The Diameter Of An Atom atom size values are calculated from atomic radius data. The covalent radius of a chlorine atom,. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. This table shows how the atom size, and atomic radius. the size of an atom can be. How To Calculate The Diameter Of An Atom.
From chem.libretexts.org
Chapter 3.2 Sizes of Atoms and Ions Chemistry LibreTexts How To Calculate The Diameter Of An Atom atom size values are calculated from atomic radius data. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. the size of an atom can be estimated by. How To Calculate The Diameter Of An Atom.
From ihsanpedia.com
How To Calculate Diameter A Comprehensive Guide IHSANPEDIA How To Calculate The Diameter Of An Atom The covalent atomic radius ( r cov ) is half. the plural of nucleus is nuclei. The covalent radius of a chlorine atom,. This table shows how the atom size, and atomic radius. atom size values are calculated from atomic radius data. a variety of methods have been established to measure the size of a single atom. How To Calculate The Diameter Of An Atom.
From sciencenotes.org
Periodic Table of Element Atom Sizes How To Calculate The Diameter Of An Atom a variety of methods have been established to measure the size of a single atom or ion. the plural of nucleus is nuclei. The covalent radius of a chlorine atom,. This table shows how the atom size, and atomic radius. the size of an atom can be estimated by measuring the distance between adjacent atoms in a. How To Calculate The Diameter Of An Atom.
From www.youtube.com
Nucleus size determination Diameter of an atom bsc physics degree How To Calculate The Diameter Of An Atom the plural of nucleus is nuclei. Atoms which have the same. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. This table shows how the atom size, and atomic radius. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. a variety. How To Calculate The Diameter Of An Atom.
From www.dreamstime.com
Atomic Radius Measurements of Diatomic Molecules Stock Vector How To Calculate The Diameter Of An Atom atom size values are calculated from atomic radius data. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom.. How To Calculate The Diameter Of An Atom.
From www.youtube.com
The diameter of zinc atom is 2.6 Å. Calculate (a) radius of zinc atom How To Calculate The Diameter Of An Atom the plural of nucleus is nuclei. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. atom size values are calculated from atomic radius data. a variety. How To Calculate The Diameter Of An Atom.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID How To Calculate The Diameter Of An Atom the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. a variety of methods have been established to measure the size of a single atom or ion. The covalent radius of a chlorine atom,. Atoms which have the same. The covalent atomic radius ( r cov ) is half.. How To Calculate The Diameter Of An Atom.
From www.numerade.com
Diameter of an Atom The diameter of an atom is about 1 ×10^10 meter How To Calculate The Diameter Of An Atom This table shows how the atom size, and atomic radius. the plural of nucleus is nuclei. the size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The covalent atomic radius ( r cov ) is half. atom size values are calculated from atomic radius data. the size. How To Calculate The Diameter Of An Atom.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent How To Calculate The Diameter Of An Atom a variety of methods have been established to measure the size of a single atom or ion. This table shows how the atom size, and atomic radius. The covalent atomic radius ( r cov ) is half. The covalent radius of a chlorine atom,. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally.. How To Calculate The Diameter Of An Atom.
From courses.lumenlearning.com
Periodic Variations in Element Properties Chemistry I How To Calculate The Diameter Of An Atom a variety of methods have been established to measure the size of a single atom or ion. Atoms which have the same. The covalent atomic radius ( r cov ) is half. atom size values are calculated from atomic radius data. This table shows how the atom size, and atomic radius. the size of an atom can. How To Calculate The Diameter Of An Atom.
From www.doubtnut.com
The diameter of an atom ranges from.. How To Calculate The Diameter Of An Atom atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. Of an atom is less than \ (\frac {1} {10,000}\) the size of an atom. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. Atoms which have the same.. How To Calculate The Diameter Of An Atom.
From sciencenotes.org
Atomic Radius and Ionic Radius How To Calculate The Diameter Of An Atom the plural of nucleus is nuclei. The covalent radius of a chlorine atom,. atomic radii can be obtained from quantum mechanical calculations or can be determined experimentally. the size of atoms can be estimated with the use of avogadro's number along with the atomic mass and bulk density of a solid. Atoms which have the same. This. How To Calculate The Diameter Of An Atom.